Application of the integrated data platform combined with dietary management for adults with diabetes: A prospective randomized controlled trial
- PMID: 39171608
- PMCID: PMC11527811
- DOI: 10.1111/jdi.14296
Application of the integrated data platform combined with dietary management for adults with diabetes: A prospective randomized controlled trial
Abstract
Aims: To investigate the efficacy of the integrated data platform of cloud hospital combined with dietary management for adults with type 2 diabetes.
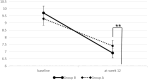
Materials and methods: We conducted a randomized controlled clinical trial. One hundred eighty patients with type 2 diabetes were randomly allocated into a control group (Group A) and an experimental group (Group B). Routine standard diabetes care was applied to the patients in Group A. The integrated data platform with dietary management was applied to Group B. Individualized diabetes education videos were sent to the patients through the platform. The primary endpoint was the change in HbA1c and change in body weight from baseline to Week 12 during the follow-up.
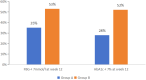
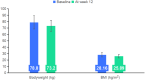
Results: At Week 12, HbA1c was 7.4 ± 0.7%, 6.9 ± 0.9% in Groups A and B, P < 0.01. The rate of fasting blood glucose <7 mmol/L, and glycosylated hemoglobin <7% was higher in Group B than in Group A. At Week 12, there was a significant weight loss and body mass index decrease in the overweight or obese patients of the experimental group. Those overweight or obese patients in the experimental group utilizing the appetite suppressant semaglutide achieved the most significant weight loss, with a 13.4% reduction after 12 weeks.
Conclusions: The integrated data platform combined with personalized diabetes education video delivery was verified to be a more effective management mode for diabetes. For overweight or obese adults with diabetes, the use of semaglutide in conjunction with dietary management and the integrated data platform led to greater weight loss.
Keywords: Dietary intervention; Digital platform; Type 2 diabetes.
© 2024 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Approval of the research protocol: This study conformed to the provisions of the Declaration of Helsinki. It was approved by the Ethics Committee of Dongyang People's Hospital.
Informed consent: All participants signed written informed consent before eligibility screening.
Registry and the registration no. of the study/trial: The approval date of registry was 30/01/2024, and the registration no. was ChiCTR2400080460.
Animal studies: N/A.
Figures
References
-
- American Diabetes Association Professional Practice Committee . 5. Facilitating behavior change and well‐being to improve health outcomes: standards of medical care in diabetes‐2022. Diabetes Care 2022; 45(Suppl 1): S60–S82. - PubMed
-
- Jia W, Weng J, Zhu D, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev 2019; 35: e3158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

