Glycerol metabolism contributes to competition by oral streptococci through production of hydrogen peroxide
- PMID: 39171915
- PMCID: PMC11411925
- DOI: 10.1128/jb.00227-24
Glycerol metabolism contributes to competition by oral streptococci through production of hydrogen peroxide
Abstract
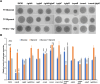
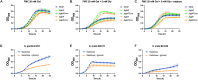
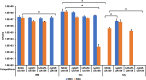
As a biological byproduct from both humans and microbes, glycerol's contribution to microbial homeostasis in the oral cavity remains understudied. In this study, we examined glycerol metabolism by Streptococcus sanguinis, a commensal associated with oral health. Genetic mutants of glucose-PTS enzyme II (manL), glycerol metabolism (glp and dha pathways), and transcriptional regulators were characterized with regard to glycerol catabolism, growth, production of hydrogen peroxide (H2O2), transcription, and competition with Streptococcus mutans. Biochemical assays identified the glp pathway as a novel source for H2O2 production by S. sanguinis that is independent of pyruvate oxidase (SpxB). Genetic analysis indicated that the glp pathway requires glycerol and a transcriptional regulator, GlpR, for expression and is negatively regulated by PTS, but not the catabolite control protein, CcpA. Conversely, deletion of either manL or ccpA increased the expression of spxB and a second, H2O2-non-producing glycerol metabolic pathway (dha), indicative of a mode of regulation consistent with conventional carbon catabolite repression (CCR). In a plate-based antagonism assay and competition assays performed with planktonic and biofilm-grown cells, glycerol greatly benefited the competitive fitness of S. sanguinis against S. mutans. The glp pathway appears to be conserved in several commensal streptococci and actively expressed in caries-free plaque samples. Our study suggests that glycerol metabolism plays a more significant role in the ecology of the oral cavity than previously understood. Commensal streptococci, though not able to use glycerol as a sole carbohydrate source for growth, benefit from the catabolism of glycerol through production of both ATP and H2O2.
Importance: Glycerol is an abundant carbohydrate in the oral cavity. However, little is understood regarding the metabolism of glycerol by commensal streptococci, some of the most abundant oral bacteria. This was in part because most streptococci cannot grow on glycerol as the sole carbon source. In this study, we show that Streptococcus sanguinis, a commensal associated with dental health, can degrade glycerol for persistence and competition through two pathways, one of which generates hydrogen peroxide at levels capable of inhibiting Streptococcus mutans. Preliminary studies suggest that several additional commensal streptococci are also able to catabolize glycerol, and glycerol-related genes are actively expressed in human dental plaque samples. Our findings reveal the potential of glycerol to significantly impact microbial homeostasis, which warrants further exploration.
Keywords: Streptococcus sanguinis; competition; dental caries; glycerol metabolism; hydrogen peroxide; phosphotransferase system (pts).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Update of
-
Glycerol Metabolism Contributes to Competition by Oral Streptococci through Production of Hydrogen Peroxide.bioRxiv [Preprint]. 2024 Jun 28:2024.06.28.598274. doi: 10.1101/2024.06.28.598274. bioRxiv. 2024. Update in: J Bacteriol. 2024 Sep 19;206(9):e0022724. doi: 10.1128/jb.00227-24. PMID: 38979179 Free PMC article. Updated. Preprint.
Similar articles
-
Systematic analysis of the glucose-PTS in Streptococcus sanguinis highlighted its importance in central metabolism and bacterial fitness.Appl Environ Microbiol. 2025 Jan 31;91(1):e0193524. doi: 10.1128/aem.01935-24. Epub 2024 Nov 25. Appl Environ Microbiol. 2025. PMID: 39584828 Free PMC article.
-
Glycerol Metabolism Contributes to Competition by Oral Streptococci through Production of Hydrogen Peroxide.bioRxiv [Preprint]. 2024 Jun 28:2024.06.28.598274. doi: 10.1101/2024.06.28.598274. bioRxiv. 2024. Update in: J Bacteriol. 2024 Sep 19;206(9):e0022724. doi: 10.1128/jb.00227-24. PMID: 38979179 Free PMC article. Updated. Preprint.
-
Glucose Phosphotransferase System Modulates Pyruvate Metabolism, Bacterial Fitness, and Microbial Ecology in Oral Streptococci.J Bacteriol. 2023 Jan 26;205(1):e0035222. doi: 10.1128/jb.00352-22. Epub 2022 Dec 5. J Bacteriol. 2023. PMID: 36468868 Free PMC article.
-
Competitive dynamics and balance between Streptococcus mutans and commensal streptococci in oral microecology.Crit Rev Microbiol. 2025 May;51(3):532-543. doi: 10.1080/1040841X.2024.2389386. Epub 2024 Aug 12. Crit Rev Microbiol. 2025. PMID: 39132685 Review.
-
Oral Commensal Streptococci: Gatekeepers of the Oral Cavity.J Bacteriol. 2022 Nov 15;204(11):e0025722. doi: 10.1128/jb.00257-22. Epub 2022 Oct 26. J Bacteriol. 2022. PMID: 36286512 Free PMC article. Review.
Cited by
-
Systematic analysis of the glucose-PTS in Streptococcus sanguinis highlighted its importance in central metabolism and bacterial fitness.Appl Environ Microbiol. 2025 Jan 31;91(1):e0193524. doi: 10.1128/aem.01935-24. Epub 2024 Nov 25. Appl Environ Microbiol. 2025. PMID: 39584828 Free PMC article.
-
Fructose activates a stress response shared by methylglyoxal and hydrogen peroxide in Streptococcus mutans.mBio. 2025 May 14;16(5):e0048525. doi: 10.1128/mbio.00485-25. Epub 2025 Apr 17. mBio. 2025. PMID: 40243330 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

