Intranasal administration of octavalent next-generation influenza vaccine elicits protective immune responses against seasonal and pre-pandemic viruses
- PMID: 39171925
- PMCID: PMC11406897
- DOI: 10.1128/jvi.00354-24
Intranasal administration of octavalent next-generation influenza vaccine elicits protective immune responses against seasonal and pre-pandemic viruses
Abstract
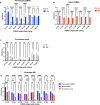
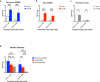
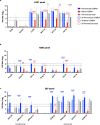
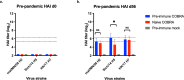
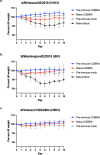
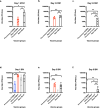
Development of next-generation influenza virus vaccines is crucial to improve protection against circulating and emerging viruses. Current vaccine formulations have to be updated annually due to mutations in seasonal strains and do not offer protection against strains with pandemic potential. Computationally optimized broadly reactive antigen (COBRA) methodology has been utilized by our group to generate broadly reactive immunogens for individual influenza subtypes, which elicit protective immune responses against a broad range of strains over numerous seasons. Octavalent mixtures of COBRA hemagglutinin (HA) (H1, H2, H3, H5, H7, and influenza B virus) plus neuraminidase (NA) (N1 and N2) recombinant proteins mixed with c-di-AMP adjuvant were administered intranasally to naive or pre-immune ferrets in prime-boost fashion. Four weeks after final vaccination, collected sera were analyzed for breadth of antibody response, and the animals were challenged with seasonal or pre-pandemic strains. The octavalent COBRA vaccine elicited antibodies that recognized a broad panel of strains representing different subtypes, and these vaccinated animals were protected against influenza virus challenges. Overall, this study demonstrated that the mixture of eight COBRA HA/NA proteins mixed with an intranasal adjuvant is a promising candidate for a universal influenza vaccine.
Importance: Influenza is a respiratory virus which infects around a billion people globally every year, with millions experiencing severe illness. Commercial vaccine efficacy varies year to year and can be low due to mismatch of circulating virus strains. Thus, the formulation of current vaccines has to be adapted accordingly every year. The development of a broadly reactive influenza vaccine would lessen the global economic and public health burden caused by the different types of influenza viruses. The significance of our research is producing a promising universal vaccine candidate which provides protection against a wider range of virus strains over a wider range of time.
Keywords: COBRA; Sting agonist; ferret; influenza; intranasal; mice; multivalent; vaccine.
Conflict of interest statement
C.A.G. and T.E. are named as inventors in patents covering the use of bis-(3,5)-cyclic dimeric adenosine monophosphate as vaccine adjuvant (PCT/EP 2006010693) and neonatal adjuvant (EP 19193982). C.A.G., T.E., N.U., and T.M.R. are named as inventors on the patent covering vaccine plus adjuvant (U.S. provisional application number 63/556,916).
Figures


Similar articles
-
A single dose of inactivated influenza virus vaccine expressing COBRA hemagglutinin elicits broadly-reactive and long-lasting protection.PLoS One. 2025 Feb 21;20(2):e0308680. doi: 10.1371/journal.pone.0308680. eCollection 2025. PLoS One. 2025. PMID: 39982912 Free PMC article.
-
Elicitation of Protective Antibodies against a Broad Panel of H1N1 Viruses in Ferrets Preimmune to Historical H1N1 Influenza Viruses.J Virol. 2017 Nov 30;91(24):e01283-17. doi: 10.1128/JVI.01283-17. Print 2017 Dec 15. J Virol. 2017. Retraction in: J Virol. 2024 Jul 23;98(7):e0183223. doi: 10.1128/jvi.01832-23. PMID: 28978709 Free PMC article. Retracted.
-
Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains.J Virol. 2021 Aug 10;95(17):e0075921. doi: 10.1128/JVI.00759-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34160258 Free PMC article.
-
Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review.
-
The Quest for a Truly Universal Influenza Vaccine.Front Cell Infect Microbiol. 2019 Oct 10;9:344. doi: 10.3389/fcimb.2019.00344. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649895 Free PMC article. Review.
Cited by
-
Single-Dose Intranasal or Intramuscular Administration of Simian Adenovirus-Based H1N1 Vaccine Induces a Robust Humoral Response and Complete Protection in Mice.Viruses. 2025 Aug 5;17(8):1085. doi: 10.3390/v17081085. Viruses. 2025. PMID: 40872799 Free PMC article.
-
Influenza A(H5N1) Immune Response among Ferrets with Influenza A(H1N1)pdm09 Immunity.Emerg Infect Dis. 2025 Mar;31(3):477-487. doi: 10.3201/eid3103.241485. Emerg Infect Dis. 2025. PMID: 40023796 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

