Management of chimeric antigen receptor T (CAR-T) cell-associated toxicities
- PMID: 39172238
- PMCID: PMC11377606
- DOI: 10.1007/s00134-024-07576-4
Management of chimeric antigen receptor T (CAR-T) cell-associated toxicities
Abstract
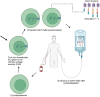
The use of chimeric antigen receptor T (CAR-T) cells is a significant therapeutic improvement increasing the prognosis for patients with a variety of hematological malignancies. However, this therapy has also sometimes life-threatening, complications. Therefore, knowledge of the treatment and management of these complications, especially in treatment centers and intensive care units, respectively, is of outstanding importance. This review provides recommendations for the diagnosis, management, and treatment of CAR-T cell-associated complications such as cytokine release syndrome, immune effector cell associated neurotoxicity syndrome, hematotoxicity, hypogammaglobulinemia, and CAR-T cell-induced pseudo-progression amongst others for physicians treating patients with CAR-T cell-associated complications and intensivists.
Keywords: CAR-T; CAR-T complications; CRS; ICAHT; ICANS; Intensive Care.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing fincancial interests or persolal relationships that could have appeared to influence the work reported in this review.
Figures



References
-
- Couzin-Frankel J (2013) Breakthrough of the year 2013. Cancer Immunotherap Sci 342(6165):1432–1433 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

