Population Pharmacokinetics and Pharmacodynamics of Dalbavancin and C-Reactive Protein in Patients with Staphylococcal Osteoarticular Infections
- PMID: 39172334
- PMCID: PMC11449996
- DOI: 10.1007/s40262-024-01410-2
Population Pharmacokinetics and Pharmacodynamics of Dalbavancin and C-Reactive Protein in Patients with Staphylococcal Osteoarticular Infections
Abstract
Background and objective: Dalbavancin is increasingly used for the long-term treatment of chronic osteoarticular infections. A population pharmacokinetic/pharmacodynamic (PK/PD) analysis for assessing the relationship between dalbavancin exposure and C-reactive protein (C-RP) over time was conducted.
Methods: Non-linear mixed-effect modeling was fitted to dalbavancin and C-RP concentrations. Monte Carlo simulations assessed the weekly percentage of C-RP reduction associated with different dosing regimens, starting from baseline to < 1 mg/dL.
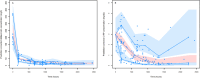
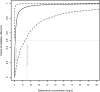
Results: A total of 45 patients were retrospectively included in the analysis. The PK of dalbavancin was described by a two-compartment model, and the PD of C-RP was described by an indirect turnover maximum inhibition model. The total dalbavancin concentration model estimate producing 50% of maximum C-RP production inhibition (IC50) was 0.70 mg/L. Monte Carlo simulations showed that in patients with staphylococcal osteoarticular infections targeting total dalbavancin concentrations at > 14.5 mg/L at any time point may achieve C-RP production inhibition over time in > 95% of patients. Based on this, the findings showed that a cumulative dose of 3000 mg administered in the first 3 weeks may lead to a > 90% C-RP decrease versus baseline in approximately 5-6 weeks. In patients needing treatment prolongation, an additional 1500 mg dose after this period may maintain C-RP concentrations < 1 mg/dL for other 3 weeks.
Conclusions: A decrease in C-RP is related to dalbavancin exposure in osteoarticular infections. Targeting dalbavancin plasma concentrations above the efficacy threshold may be associated with effective treatment.
© 2024. The Author(s).
Conflict of interest statement
Pier Giorgio Cojutti received fees from Angelini, Shionogi, Pfizer, and MSD outside of the submitted work. Federico Pea reports personal fees from Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, Thermo Fisher, and Accelerate Diagnostics, outside the submitted work; has participated in speakers’ bureaus for Accelerate Diagnostics, Angelini, Basilea Pharmaceutica, Gilead, Hikma, MSD, Pfizer, Sanofi-Aventis, Shionogi, and Thermo Fisher; and has been a consultant for Angelini, Basilea Pharmaceutica, Gilead, MSD, Pfizer, and Shionogi, outside the submitted work. Pierluigi Viale has served as a consultant for bioMérieux, Gilead, Merck Sharp and Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and has received payment for serving on the speakers’ bureaus for Correvio, Gilead, Merck Sharp, and Dohme, Nordic Pharma, and Pfizer, outside the submitted work. Sara Tedeschi and Eleonora Zamparini report no potential conflicts of interest that may be relevant to the contents of this study.
Figures


References
-
- Cojutti PG, Tedeschi S, Gatti M, Zamparini E, Meschiari M, Siega PD, et al. Population pharmacokinetic and pharmacodynamic analysis of dalbavancin for long-term treatment of subacute and/or chronic infectious diseases: the major role of therapeutic drug monitoring. Antibiotics. 2022;11(8):996. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

