Elinzanetant for the Treatment of Vasomotor Symptoms Associated With Menopause: OASIS 1 and 2 Randomized Clinical Trials
- PMID: 39172446
- PMCID: PMC11342219
- DOI: 10.1001/jama.2024.14618
Elinzanetant for the Treatment of Vasomotor Symptoms Associated With Menopause: OASIS 1 and 2 Randomized Clinical Trials
Abstract
Importance: Safe and effective nonhormonal treatments for menopausal vasomotor symptoms (VMS) are needed.
Objective: To evaluate the efficacy and safety of elinzanetant, a selective neurokinin-1,3 receptor antagonist, for the treatment of moderate to severe menopausal vasomotor symptoms.
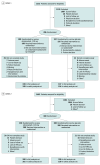
Design, setting, and participants: Two randomized double-blind phase 3 trials (OASIS 1 and 2) included postmenopausal participants aged 40 to 65 years experiencing moderate to severe vasomotor symptoms (OASIS 1: 77 sites in the US, Europe, and Israel from August 27, 2021, to November 27, 2023, and OASIS 2: 77 sites in the US, Canada, and Europe from October 29, 2021, to October 10, 2023).
Intervention: Once daily oral elinzanetant, 120 mg, for 26 weeks or matching placebo for 12 weeks followed by elinzanetant, 120 mg, for 14 weeks.
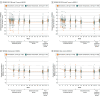
Main outcomes and measures: Primary end points included mean change in frequency and severity of moderate to severe vasomotor symptoms from baseline to weeks 4 and 12, measured by the electronic hot flash daily diary. Secondary end points included Patient-Reported Outcomes Measurement Information System Sleep Disturbance Short Form 8b total T score and Menopause-Specific Quality of Life questionnaire total score from baseline to week 12.
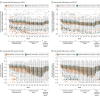
Results: Eligible participants (mean [SD] age, OASIS 1: 54.6 [4.9] years; OASIS 2: 54.6 [4.8] years) were randomized to elinzanetant (OASIS 1: n = 199; OASIS 2: n = 200) or placebo (OASIS 1: n = 197; OASIS 2: n = 200). A total of 309 (78.0%) and 324 (81.0%) completed OASIS 1 and 2, respectively. For the elinzanetant and placebo groups, the baseline mean (SD) VMS per 24 hours were 13.4 (6.6) vs 14.3 (13.9) (OASIS 1) and 14.7 (11.1) v 16.2 (11.2) (OASIS 2). Baseline VMS severity was 2.6 (0.2) vs 2.5 (0.2) (OASIS 1) and 2.5 (0.2) vs 2.5 (0.2) (OASIS 2). Elinzanetant significantly reduced VMS frequency at week 4 (OASIS 1: -3.3 [95% CI, -4.5 to -2.1], P < .001; OASIS 2: -3.0 [95% CI, -4.4 to -1.7], P < .001) and at week 12 (OASIS 1: -3.2 [95% CI, -4.8 to -1.6], P < .001; OASIS 2: -3.2 [95% CI, -4.6 to -1.9], P < .001). Elinzanetant also improved VMS severity at week 4 (OASIS 1: -0.3 [95% CI, -0.4 to -0.2], P < .001; OASIS 2: -0.2 [95 CI, -0.3 to -0.1], P < .001) and week 12 (OASIS 1: -0.4 [95% CI, -0.5 to -0.3], P < .001; OASIS 2: -0.3 [95% CI, -0.4 to -0.1], P < .001). Elinzanetant improved sleep disturbances and menopause-related quality of life at week 12, and the safety profile was favorable.
Conclusions and relevance: Elinzanetant was well tolerated and efficacious for moderate to severe menopausal VMS.
Trial registration: ClinicalTrials.gov Identifier: OASIS 1: NCT05042362, OASIS 2: NCT05099159.
Conflict of interest statement
Figures
Comment in
References
Associated data
LinkOut - more resources
Full Text Sources
Medical

