Efficacy and Safety of Pimodivir Combined With Standard of Care in Hospitalized and Nonhospitalized High-Risk Adolescents and Adults With Influenza A Infection
- PMID: 39172627
- PMCID: PMC11793051
- DOI: 10.1093/infdis/jiae408
Efficacy and Safety of Pimodivir Combined With Standard of Care in Hospitalized and Nonhospitalized High-Risk Adolescents and Adults With Influenza A Infection
Abstract
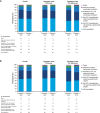
Background: An unmet need exists for effective antivirals to treat patients hospitalized with influenza. The results of 2 phase 3 studies that evaluated the efficacy and safety of pimodivir in combination with investigator-chosen standard of care (SoC) treatment are presented.
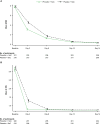
Methods: Hospitalized patients (hospital study; NCT03376321) and high-risk outpatients (outpatient study; NCT03381196) with laboratory-confirmed influenza A infection were randomized 1:1 to 600 mg pimodivir twice daily + SoC or placebo twice daily + SoC for 5 days. For most patients, SoC included oseltamivir. Primary end points were Hospital Recovery Scale (HRS) at day 6 (hospital study) and median time to resolution (TTR) of influenza-related symptoms (outpatient study).
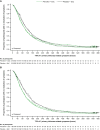
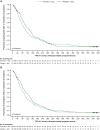
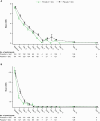
Results: Pimodivir + SoC (oseltamivir) treatment showed no clinical benefit over placebo + SoC on HRS at day 6 (common odds ratio, 0.943; 95% confidence interval [CI], .609-1.462; P = .397; hospital study). A shorter median TTR of 7 symptoms was estimated with pimodivir + SoC versus placebo (92.6 hours; 95% CI, 77.6-104.2 vs 105.1 hours; 95% CI, 92.7-128.6; P = .0216; outpatient study).
Conclusions: Pimodivir + SoC showed no additional clinical benefit versus SoC treatment alone in hospitalized patients. Pimodivir + SoC demonstrated shorter TTR of influenza symptoms versus placebo + SoC in high-risk outpatients.
Clinical trial registration: NCT03376321 and NCT03381196.
Keywords: Hospital Recovery Scale; hospitalized patients; influenza A; ordinal endpoint; respiratory viral infection.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. L. L., J. V., S. D., C. N., I. V. D., B. M., and W. V. D. are employees of Janssen (part of Johnson & Johnson) and stockholders of Johnson & Johnson. K. W. was an employee of Janssen (part of Johnson & Johnson) at the time of writing and is a stockholder of Johnson & Johnson and Kenvue, Inc. D. L. is an employee of Janssen (part of Johnson & Johnson). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Centers for Disease Control and Prevention . Key facts about influenza (Flu), 2022. https://www.cdc.gov/flu/about/keyfacts.htm#print. Accessed 18 October 2023.
-
- Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

