PIONEER REAL Japan: Primary results from a multicenter, prospective, real-world study of oral semaglutide in adults with type 2 diabetes in Japanese clinical practice
- PMID: 39172634
- PMCID: PMC11527839
- DOI: 10.1111/jdi.14291
PIONEER REAL Japan: Primary results from a multicenter, prospective, real-world study of oral semaglutide in adults with type 2 diabetes in Japanese clinical practice
Abstract
Aims/introduction: PIONEER REAL Japan was a non-interventional prospective study of oral semaglutide in adults with type 2 diabetes in Japanese clinical practice.
Materials and methods: Adults naïve to injectable glucose-lowering therapies initiated oral semaglutide in routine clinical practice and were followed for 34-44 weeks. The primary endpoint was change in glycated hemoglobin (HbA1c) from baseline to end of study; the co-primary endpoint was number of adverse events (AEs). Secondary endpoints included change in bodyweight from baseline to end of study. Analyses were also carried out for subgroups aged <75 and ≥75 years.
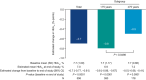
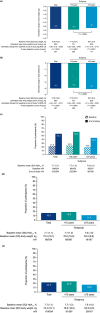
Results: A total of 624 participants initiated oral semaglutide; 578 completed the study. Mean baseline HbA1c and bodyweight were 7.7% and 72.4 kg, respectively. At end of study, estimated change (95% confidence interval [CI]) in HbA1c from baseline was -0.7 percentage points (-0.77, -0.61) overall, -0.8 percentage points (-0.86, -0.67) in the <75 years subgroup and -0.5 percentage points (-0.68, -0.41) in the ≥75 years subgroup (all P < 0.0001). Estimated change (95% CI) in bodyweight was -2.8 (-3.19, -2.50) kg overall, -2.9 (-3.38, -2.49) kg in the <75 years subgroup and - 2.7 (-3.18, -2.14) kg in the ≥75 years subgroup (all P < 0.0001). AEs occurred in 161 (25.8%) participants: 99 of 423 (23.4%) and 62 of 201 (30.8%) participants in the <75 and ≥75 years subgroups, respectively. Gastrointestinal AEs were the AEs most frequently leading to oral semaglutide discontinuation.
Conclusions: In routine clinical practice, HbA1c and bodyweight were significantly reduced from baseline in adults initiating oral semaglutide, including those aged ≥75 years, with no new safety concerns.
Keywords: Diabetes mellitus, type 2; Oral semaglutide; Prospective studies.
© 2024 The Author(s). Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Daisuke Yabe received consulting/lecture fees from Eli Lilly Japan K.K., Kyowa Kirin Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Sanofi K.K. and Sumitomo Pharma Co., Ltd.; and research funding/grants from Arkray Inc., Nippon Boehringer Ingelheim Co., Ltd., Novo Nordisk Pharma Ltd., Taisho Pharmaceutical Co., Ltd. and Terumo Corporation. Professor Yabe is an Editorial Board member of
Approval of the research protocol: The protocol for this research project has been approved by suitably constituted ethics committees or institutional review boards of the institutions, and it conforms to the provisions of the Declaration of Helsinki. Ethics committees, institutional review boards and approval numbers are listed in the Supporting Information.
Informed consent: All informed consent was obtained from the participants and/or their guardians.
Approval date of registry and the registration no. of the study/trial: This study is registered with
Animal studies: N/A.
Figures

References
-
- International Diabetes Federation . IDF Diabetes Atlas, 10th edn. Brussels, Belgium: International Diabetes Federation, 2021. Available from: https://diabetesatlas.org/idfawp/resource‐files/2021/07/IDF_Atlas_10th_E... Accessed January 30, 2024.
-
- Miyazawa I, Yokoyama H, Yagi N, et al. Annual trends in glycemic control and prescribing patterns in diabetic treatment according to age in Japanese patients with type 2 diabetes between 2012 and 2019 (JDDM 71). Diabetes Res Clin Pract 2023; 198: 110599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

