Protocol for quantifying drug sensitivity in 3D patient-derived ovarian cancer models
- PMID: 39172645
- PMCID: PMC11387699
- DOI: 10.1016/j.xpro.2024.103274
Protocol for quantifying drug sensitivity in 3D patient-derived ovarian cancer models
Abstract
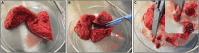
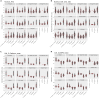
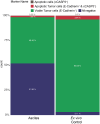
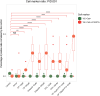
Three-dimensional (3D) ex vivo cultures allow the study of cancer progression and drug resistance mechanisms. Here, we present a protocol for measuring on-target drug sensitivity in a scaffold-free 3D culture system through quantification of apoptotic tumor cells. We provide detailed steps for sample processing, immunofluorescence staining, semi-high-throughput confocal imaging, and imaged-based quantification of 3D cultures. This protocol is versatile and can be applied in principle to any patient-derived material.
Keywords: Cancer; Cell culture; Single Cell.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures








References
-
- Song H., Cai G.-H., Liang J., Ao D.-S., Wang H., Yang Z.-H. Three-dimensional culture and clinical drug responses of a highly metastatic human ovarian cancer HO-8910PM cells in nanofibrous microenvironments of three hydrogel biomaterials. J. Nanobiotechnology. 2020;18:90. doi: 10.1186/s12951-020-00646-x. - DOI - PMC - PubMed
-
- Golan S., Bar V., Salpeter S.J., Neev G., Creiderman G., Kedar D., Aharon S., Turovsky L., Zundelevich A., Shahar H., et al. A clinical evaluation of an ex vivo organ culture system to predict patient response to cancer therapy. Front. Med. 2023;10 doi: 10.3389/fmed.2023.1221484. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

