Preclinical Characterization of ARX517, a Site-Specific Stable PSMA-Targeted Antibody-Drug Conjugate for the Treatment of Metastatic Castration-Resistant Prostate Cancer
- PMID: 39172730
- PMCID: PMC11612621
- DOI: 10.1158/1535-7163.MCT-23-0927
Preclinical Characterization of ARX517, a Site-Specific Stable PSMA-Targeted Antibody-Drug Conjugate for the Treatment of Metastatic Castration-Resistant Prostate Cancer
Abstract
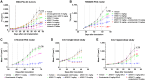
Metastatic castration-resistant prostate cancer (mCRPC) is an advanced disease in which patients ultimately fail standard-of-care androgen deprivation therapies and exhibit poor survival rates. The prostate-specific membrane antigen (PSMA) has been validated as an mCRPC tumor antigen with overexpression in tumors and low expression in healthy tissues. Using our proprietary technology for incorporating synthetic amino acids into proteins at selected sites, we have developed ARX517, an antibody-drug conjugate composed of a humanized anti-PSMA antibody site-specifically conjugated to a tubulin inhibitor at a drug-to-antibody ratio of 2. After binding PSMA, ARX517 is internalized and catabolized, leading to cytotoxic payload delivery and apoptosis. To minimize premature payload release and maximize delivery to tumor cells, ARX517 employs a noncleavable polyethylene glycol linker and stable oxime conjugation enabled via synthetic amino acid protein incorporation to ensure its overall stability. In vitro studies demonstrate that ARX517 selectively induces cytotoxicity of PSMA-expressing tumor cell lines. ARX517 exhibited a long terminal half-life and high serum exposure in mice and dose-dependent antitumor activity in both enzalutamide-sensitive and -resistant cell line-derived xenograft and patient-derived xenograft models of prostate cancer. Repeat-dose toxicokinetic studies in nonhuman primates demonstrated that ARX517 was tolerated at exposures well above therapeutic exposures in mouse pharmacology studies, indicating a wide therapeutic index. In summary, ARX517 inhibited tumor growth in diverse mCRPC models, demonstrated a tolerable safety profile in monkeys, and had a wide therapeutic index based on preclinical exposure data. Based on the encouraging preclinical data, ARX517 is currently being evaluated in a phase I clinical trial (NCT04662580).
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
All authors were employees of Ambrx at the time the work was conducted. L.K. Skidmore reports a patent for US11420999 issued to Ambrx, Inc. N.A. Knudsen reports a patent for 20220033518 A1 pending. F. Tian reports a patent for US10800856 issued. No disclosures were reported by the other authors.
Figures



References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17–48. - PubMed
-
- Lowrance W, Dreicer R, Jarrard DF, Scarpato KR, Kim SK, Kirkby E, et al. Updates to advanced prostate cancer: AUA/SUO guideline (2023). J Urol 2023;209:1082–90. - PubMed
-
- Queisser A, Hagedorn SA, Braun M, Vogel W, Duensing S, Perner S. Comparison of different prostatic markers in lymph node and distant metastases of prostate cancer. Mod Pathol 2015;28:138–45. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

