Multiscale modeling shows how 2'-deoxy-ATP rescues ventricular function in heart failure
- PMID: 39172779
- PMCID: PMC11363293
- DOI: 10.1073/pnas.2322077121
Multiscale modeling shows how 2'-deoxy-ATP rescues ventricular function in heart failure
Abstract
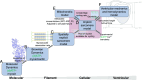
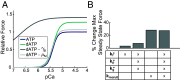
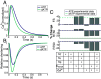
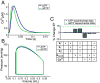
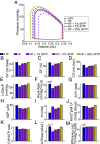
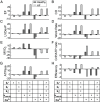
2'-deoxy-ATP (dATP) improves cardiac function by increasing the rate of crossbridge cycling and Ca[Formula: see text] transient decay. However, the mechanisms of these effects and how therapeutic responses to dATP are achieved when dATP is only a small fraction of the total ATP pool remain poorly understood. Here, we used a multiscale computational modeling approach to analyze the mechanisms by which dATP improves ventricular function. We integrated atomistic simulations of prepowerstroke myosin and actomyosin association, filament-scale Markov state modeling of sarcomere mechanics, cell-scale analysis of myocyte Ca[Formula: see text] dynamics and contraction, organ-scale modeling of biventricular mechanoenergetics, and systems level modeling of circulatory dynamics. Molecular and Brownian dynamics simulations showed that dATP increases the actomyosin association rate by 1.9 fold. Markov state models predicted that dATP increases the pool of myosin heads available for crossbridge cycling, increasing steady-state force development at low dATP fractions by 1.3 fold due to mechanosensing and nearest-neighbor cooperativity. This was found to be the dominant mechanism by which small amounts of dATP can improve contractile function at myofilament to organ scales. Together with faster myocyte Ca[Formula: see text] handling, this led to improved ventricular contractility, especially in a failing heart model in which dATP increased ejection fraction by 16% and the energy efficiency of cardiac contraction by 1%. This work represents a complete multiscale model analysis of a small molecule myosin modulator from single molecule to organ system biophysics and elucidates how the molecular mechanisms of dATP may improve cardiovascular function in heart failure with reduced ejection fraction.
Keywords: cardiac function; dATP; multiscale modeling; myosin; sarcomere.
Conflict of interest statement
Competing interests statement:A.D.M. is a cofounder of and has equity interests in Insilicomed Inc. and Vektor Medical, Inc. He serves as scientific advisor to both companies. Some of A.D.M.’s research grants have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Insilicomed Inc. and Vektor Medical, Inc. The author is required to disclose this relationship in publications acknowledging the grant support; however, the research subject and findings reported in this study did not involve the companies in any way and have no relationship with the business activities or scientific interests of either company. The terms of this arrangement have been reviewed and approved by the University of California San Diego in accordance with its conflict of interest policies.
Figures

References
-
- Regnier M., Rivera A. J., Chen Y., Chase P. B., 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ. Res. 86, 1211–1217 (2000). - PubMed
MeSH terms
Substances
Grants and funding
- HL137100/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- HL128368/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- TG-MCB200100/National Science Foundation (NSF)
- P30 AR074990/AR/NIAMS NIH HHS/United States
- T32 HL007828/HL/NHLBI NIH HHS/United States
- R01 HL173346/HL/NHLBI NIH HHS/United States
- R01 GM031749/GM/NIGMS NIH HHS/United States
- T32HL007828/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- ACI-1548562/National Science Foundation (NSF)
- R01 HL128368/HL/NHLBI NIH HHS/United States
- P41GM103426/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 HL154624/HL/NHLBI NIH HHS/United States
- GM31749/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- HL154624/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL137100/HL/NHLBI NIH HHS/United States
- 906494/American Heart Association (AHA)
- P41 GM103426/GM/NIGMS NIH HHS/United States
- T32 HL007444/HL/NHLBI NIH HHS/United States
- DGE-2038238/NSF | National Science Foundation Graduate Research Fellowship Program (GRFP)
LinkOut - more resources
Full Text Sources
Medical

