Evaluating research ethics committees in Vietnam and Laos: Results of a validated self-assessment tool
- PMID: 39172804
- PMCID: PMC11340885
- DOI: 10.1371/journal.pone.0309084
Evaluating research ethics committees in Vietnam and Laos: Results of a validated self-assessment tool
Abstract
Background: There is an increase in human subject research in developing countries and conducting them in an ethical manner depends on the research ethics oversight in these countries. The purpose of this study is to evaluate the operational, financial, and educational characteristics of research ethics committees (RECs) at institutions in Vietnam and Laos.
Methods: A validated self-assessment tool designed to assess nine major characteristics of RECs was translated into Vietnamese and Laotian. The translated surveys were delivered to and completed by representatives from RECs at institutions in Vietnam and Laos. The surveys were collected, translated back into English, and scored. The data was analyzed to identify potential areas of strength and areas for improvement.
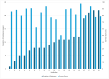
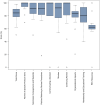
Results: The mean survey score for the 19 RECs surveyed was 165.3 out of a maximum of 200 points with a standard deviation of 22.9. Committees scored the highest in the review of specific protocol items (95.6%), submission arrangements and materials (89.5%), and the policies referring to review procedures (85.6%) domains. RECs scored the lowest in the resources domain (65.5%), with only 26.3% of committees having an annual budget. Nearly all RECs have standard operating procedures (94.7%) and policies for disclosing conflicts of interest (89.5%). Most committees use prior ethics training as a criterion to select REC chairs (78.9%) and members (73.7%), with the majority of committees requiring a training course in ethics (76.5%). 68.4% of committees have continuing education in ethics for members and only 42.1% of committees have a budget for member training.
Conclusion: This study demonstrated that RECs in Vietnam and Laos have strong foundational review processes for research protocols. Important areas of improvement include improved institutional oversight, financial and administrative resources, and the continued ethics education for current committee members.
Copyright: © 2024 Sattah et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Wang X, Hahne J, Li L, Khoshnood K, Yang G, Yuan H, et al. Developing Quality and Efficiency of Institutional Review Board Review Under a Human Research Protection Program at a Leading Hospital in Central Southern China: A Descriptive Analysis of the First Three Years. J Empir Res Hum Res Ethics. 2021;16(3):280–9. doi: 10.1177/1556264621995656 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources