Investigation of dermal collagen nanostructures in Ehlers-Danlos Syndrome (EDS) patients
- PMID: 39172992
- PMCID: PMC11341037
- DOI: 10.1371/journal.pone.0307442
Investigation of dermal collagen nanostructures in Ehlers-Danlos Syndrome (EDS) patients
Abstract
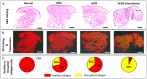
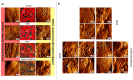
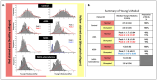
Ehlers-Danlos syndromes (EDS) represent a group of rare genetic disorders affecting connective tissues. Globally, approximately 1.5 million individuals suffer from EDS, with 10,000 reported cases in Canada alone. Understanding the histological properties of collagen in EDS has been challenging, but advanced techniques like atomic force microscopy (AFM) have opened up new possibilities for label-free skin imaging. This approach, which explores Type I collagen fibrils at the nanoscale, could potentially enhance EDS diagnosis and our knowledge of collagen type I-related connective tissue disorders. In the current study, we have employed AFM to examine ex-vivo skin biopsies from four individuals: one with classical EDS (cEDS), one with hypermobile EDS (hEDS), one with hEDS and Scleroderma (hEDS-Scleroderma), and one healthy control. Picrosirius red (PS) staining was used to highlight collagen differences in the samples. For each case, 14 images and 1400 force curves were obtained, with seven images and 700 force curves representing healthy collagen (PS-induced red staining) and the rest showcasing disrupted collagen (yellow staining). The results showed that PS staining was uniform throughout the control section, while cEDS and hEDS displayed localized areas of yellow staining. In the case of hEDS-Scleroderma, the yellow staining was widespread throughout the section. AFM images revealed irregular collagen fibrils in the disrupted, yellow-stained areas, contrasting with aligned and well-registered collagen fibrils in healthy, red-stained regions. Additionally, the study assessed the ability of non-AFM specialists to differentiate between healthy and disrupted collagen in AFM images, yielding substantial agreement among raters according to Fleiss's and Cohen's kappa scores (0.96 and 0.79±0.1, respectively). Biomechanical analysis revealed that normal healthy collagen exhibited a predominant population at 2.5 GPa. In contrast, EDS-affected collagen displayed subpopulations with lower compressive elastic modulus, indicating weaker collagen fibrils in EDS patients. Although these findings pertain to a limited number of cases, they offer valuable insights into the nanoscale collagen structure and biomechanics in individuals with EDS. Over time, these insights could be developed into specific biomarkers for the condition, improving diagnosis and treatment for EDS and related connective tissue disorders.
Copyright: © 2024 Neshatian et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Miller E, Grosel JM. A review of Ehlers-Danlos syndrome. JAAPA. 2020;33: 23–28. - PubMed
-
- Demmler JC, Atkinson MD, Reinhold EJ, Choy E, Lyons RA, Brophy ST. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case–control comparison. BMJ open. 2019;9: e031365. doi: 10.1136/bmjopen-2019-031365 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

