One-year experience with latanoprostene bunod ophthalmic solution 0.024% in clinical practice: A retrospective observational study
- PMID: 39173013
- PMCID: PMC11341023
- DOI: 10.1371/journal.pone.0307132
One-year experience with latanoprostene bunod ophthalmic solution 0.024% in clinical practice: A retrospective observational study
Abstract
Purpose: We evaluated the IOP-lowering efficacy and safety of latanoprostene bunod (LBN) ophthalmic solution 0.024% (Vyzulta®), the first topical nitric oxide-donating prostaglandin analog (PGA), in clinical practice.
Materials and methods: A retrospective medical chart review from July 2021 to July 2023 of patients with open-angle glaucoma receiving LBN with at least 1 year follow-up was conducted. All included patients received LBN 0.024% as a replacement for a PGA, with examinations at 1-, 3-, 6-and 12-months follow-up. Main outcome measures were IOP, retinal nerve fiber layer thickness, visual fields before/after LBN use and adverse effects. Subgroup analysis with glaucoma types and PGA use were performed for additional IOP reduction after LBN use.
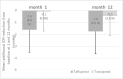
Results: Among 78 included patients, 47 patients (81 eyes), 60% with open-angle glaucoma (OAG) remained on LBN throughout 12-month follow-up. Baseline IOP was 18.2±4.2 mm Hg, and Prostaglandin analog (PGA)-IOP was 14.4 ± 3.0 mm Hg (21% mean IOP reduction). After switched to LBN, mean additional IOP reduction was 1.0 mm Hg at month 1, and the greatest reduction was 1.6 mm Hg (8.8% additional mean IOP reduction) at month 12 (P<0.0001). Subgroup analysis (NTG, 73%) showed that mean additional IOP reduction at month 12 was 1.3±2.0 mm Hg in NTG group and 2.1±3.2 mm Hg in POAG group (7.7% vs. 8.7% additional IOP reduction rates, P = 0.23). Subgroup analysis of PGA use at month 12 was 1.8±2.3 mm Hg in tafluoprost group and 0.5±1.7 mm Hg in travoprost group (9.5% vs.2.6% additional IOP reduction rates, P = 0.02). Tolerable ocular adverse effects included irritation (n = 16, 19.8%), mild conjunctival hyperemia (n = 11, 13.6%), dark circles (n = 4, 4.9%) and blurred vision (n = 2, 2.5%). There were no significant visual field and retinal nerve fiber layer thickness changes after 12 months of treatment with LBN 0.024%.
Conclusions: Although high intolerable adverse effects including conjunctival hyperemia and eye irritation happened in the first month, remaining sixty percent of patients exhibited statistically significant additional IOP reductions in the replacement of other PGAs during 12 months of clinical use of LBN 0.024%.
Copyright: © 2024 Hsueh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Prum BE Jr., Rosenberg LF, Gedde SJ, Mansberger SL, Stein JD, Moroi SE, et al.. Primary Open-Angle Glaucoma Preferred Practice Pattern(®) Guidelines. Ophthalmology. 2016;123(1):P41–p111. doi: 10.1016 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

