Phase III KEYNOTE-789 Study of Pemetrexed and Platinum With or Without Pembrolizumab for Tyrosine Kinase Inhibitor‒Resistant, EGFR-Mutant, Metastatic Nonsquamous Non-Small Cell Lung Cancer
- PMID: 39173098
- PMCID: PMC11608596
- DOI: 10.1200/JCO.23.02747
Phase III KEYNOTE-789 Study of Pemetrexed and Platinum With or Without Pembrolizumab for Tyrosine Kinase Inhibitor‒Resistant, EGFR-Mutant, Metastatic Nonsquamous Non-Small Cell Lung Cancer
Abstract
Purpose: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are standard first-line therapy for EGFR-mutant, metastatic non-small cell lung cancer (NSCLC); however, most patients experience disease progression. We report results from the randomized, double-blind, phase III KEYNOTE-789 study of pemetrexed and platinum-based chemotherapy with or without pembrolizumab for TKI-resistant, EGFR-mutant, metastatic nonsquamous NSCLC (ClinicalTrials.gov identifier: NCT03515837).
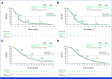
Methods: Adults with pathologically confirmed stage IV nonsquamous NSCLC, documented DEL19 or L858R EGFR mutation, and progression after EGFR-TKI treatment were randomly assigned 1:1 to 35 cycles of pembrolizumab 200 mg or placebo once every 3 weeks plus four cycles of pemetrexed and carboplatin or cisplatin once every 3 weeks and then maintenance pemetrexed. Dual primary end points were progression-free survival (PFS) and overall survival (OS). Final PFS testing was completed at the second interim analysis (IA2; data cutoff, December 3, 2021); OS was tested at final analysis (FA; data cutoff, January 17, 2023). Efficacy boundaries were one-sided P = .0117 for PFS and OS.
Results: Four hundred ninety-two patients were randomly assigned to pembrolizumab plus chemotherapy (n = 245) or placebo plus chemotherapy (n = 247). At IA2, the median PFS was 5.6 months for pembrolizumab plus chemotherapy versus 5.5 months for placebo plus chemotherapy (hazard ratio [HR], 0.80 [95% CI, 0.65 to 0.97]; P = .0122). At FA, the median OS was 15.9 versus 14.7 months, respectively (HR, 0.84 [95% CI, 0.69 to 1.02]; P = .0362). Grade ≥3 treatment-related adverse events occurred in 43.7% of pembrolizumab plus chemotherapy recipients versus 38.6% of placebo plus chemotherapy recipients.
Conclusion: Addition of pembrolizumab to chemotherapy in patients with TKI-resistant, EGFR-mutant, metastatic nonsquamous NSCLC did not significantly prolong PFS or OS versus placebo plus chemotherapy in KEYNOTE-789.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures




References
-
- Shields MD, Marin-Acevedo JA, Pellini B: Immunotherapy for advanced non-small cell lung cancer: A decade of progress. Am Soc Clin Oncol Educ Book 41:1-23, 2021 - PubMed
-
- Hendriks LE, Kerr KM, Menis J, et al. : Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 34:339-357, 2023 - PubMed
-
- Lee CK, Man J, Lord S, et al. : Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer—A meta-analysis. J Thorac Oncol 12:403-407, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

