Vital Signs: Trends and Disparities in Childhood Vaccination Coverage by Vaccines for Children Program Eligibility - National Immunization Survey-Child, United States, 2012-2022
- PMID: 39173180
- PMCID: PMC11349383
- DOI: 10.15585/mmwr.mm7333e1
Vital Signs: Trends and Disparities in Childhood Vaccination Coverage by Vaccines for Children Program Eligibility - National Immunization Survey-Child, United States, 2012-2022
Abstract
Introduction: The Vaccines for Children (VFC) program was established in 1994 to provide recommended vaccines at no cost to eligible children and help ensure that all U.S. children are protected from life-threatening vaccine-preventable diseases.
Methods: CDC analyzed data from the 2012-2022 National Immunization Survey-Child (NIS-Child) to assess trends in vaccination coverage with ≥1 dose of measles, mumps, and rubella vaccine (MMR), 2-3 doses of rotavirus vaccine, and a combined 7-vaccine series, by VFC program eligibility status, and to examine differences in coverage among VFC-eligible children by sociodemographic characteristics. VFC eligibility was defined as meeting at least one of the following criteria: 1) American Indian or Alaska Native; 2) insured by Medicaid, Indian Health Service (IHS), or uninsured; or 3) ever received at least one vaccination at an IHS-operated center, Tribal health center, or urban Indian health care facility.
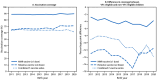
Results: Overall, approximately 52.2% of U.S. children were VFC eligible. Among VFC-eligible children born during 2011-2020, coverage by age 24 months was stable for ≥1 MMR dose (88.0%-89.9%) and the combined 7-vaccine series (61.4%-65.3%). Rotavirus vaccination coverage by age 8 months was 64.8%-71.1%, increasing by an average of 0.7 percentage points annually. Among all children born in 2020, coverage was 3.8 (≥1 MMR dose), 11.5 (2-3 doses of rotavirus vaccine), and 13.8 (combined 7-vaccine series) percentage points lower among VFC-eligible than among non-VFC-eligible children.
Conclusions and implications for public health practice: Although the VFC program has played a vital role in increasing and maintaining high levels of childhood vaccination coverage for 30 years, gaps remain. Enhanced efforts must ensure that parents and guardians of VFC-eligible children are aware of, have confidence in, and are able to obtain all recommended vaccines for their children.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
References
-
- Zhou F, Jataloui TC, Leidner AJ, et al.. Health and economic benefits of routine childhood immunizations in the era of the Vaccines for Children program—United States, 1994–2023. MMWR Morb Mortal Wkly Rep 2024;73:682–5. https://www.cdc.gov/mmwr/volumes/73/wr/mm7331a2.htm?s_cid=mm7331a2_w - PMC - PubMed
-
- CDC. Vaccines for Children (VFC) program operations guide. Atlanta, GA: US Department of Health and Human Services, CDC; 2024. Accessed August 5, 2024. https://www.cdc.gov/vaccines-for-children/downloads/operations-guide-508...
-
- Walker AT, Smith PJ, Kolasa M; CDC. Reduction of racial/ethnic disparities in vaccination coverage, 1995-2011. MMWR Suppl 2014;63:7–12. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

