Plin4 exacerbates cadmium-decreased testosterone level via inducing ferroptosis in testicular Leydig cells
- PMID: 39173539
- PMCID: PMC11387904
- DOI: 10.1016/j.redox.2024.103312
Plin4 exacerbates cadmium-decreased testosterone level via inducing ferroptosis in testicular Leydig cells
Abstract
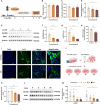
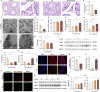
Strong evidence indicates that environmental stressors are the risk factors for male testosterone deficiency (TD). However, the mechanisms of environmental stress-induced TD remain unclear. Based on our all-cause male reproductive cohort, we found that serum ferrous iron (Fe2⁺) levels were elevated in TD donors. Then, we explored the role and mechanism of ferroptosis in environmental stress-reduced testosterone levels through in vivo and in vitro models. Data demonstrated that ferroptosis and lipid droplet deposition were observed in environmental stress-exposed testicular Leydig cells. Pretreatment with ferrostatin-1 (Fer-1), a specific ferroptosis inhibitor, markedly mitigated environmental stress-reduced testosterone levels. Through screening of core genes involved in lipid droplets formation, it was found that environmental stress significantly increased the levels of perilipins 4 (PLIN4) protein and mRNA in testicular Leydig cells. Further experiments showed that Plin4 siRNA reversed environmental stress-induced lipid droplet deposition and ferroptosis in Leydig cells. Additionally, environmental stress increased the levels of METTL3, METTL14, and total RNA m6A in testicular Leydig cells. Mechanistically, S-adenosylhomocysteine, an inhibitor of METTL3 and METTL14 heterodimer activity, restored the abnormal levels of Plin4, Fe2⁺ and testosterone in environmental stress-treated Leydig cells. Collectively, these results suggest that Plin4 exacerbates environmental stress-decreased testosterone level via inducing ferroptosis in testicular Leydig cells.
Keywords: Environment stress; Ferroptosis; Lipid droplet; Perilipins; Testosterone deficiency; m6A modification.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Dean J.D., McMahon C.G., Guay A.T., Morgentaler A., Althof S.E., Becher E.F., et al. The isociety for sexual medicine's process of care for the assessment and management of testosterone deficiency in adult men. J. Sex. Med. 2015;12:1660–1686. - PubMed
-
- Khera M., Adaikan G., Buvat J., Carrier S., El-Meliegy A., Hatzimouratidis K., et al. Diagnosis and treatment of testosterone deficiency: recommendations from the fourth international consultation for sexual medicine (ICSM 2015) J. Sex. Med. 2016;13:1787–1804. - PubMed
-
- Tajar A., Forti G., O'Neill T.W., Lee D.M., Silman A.J., Finn J.D., et al. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J. Clin. Endocrinol. Metabol. 2010;95:1810–1818. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

