Impact of primary tumor sidedness and sex on prognosis and anti-epidermal growth factor receptor antibody efficacy in BRAF-mutant metastatic colorectal cancer: a pooled analysis of AIO studies FIRE-1, CIOX, FIRE-3, XELAVIRI, and VOLFI
- PMID: 39173562
- PMCID: PMC11387224
- DOI: 10.1016/j.esmoop.2024.103677
Impact of primary tumor sidedness and sex on prognosis and anti-epidermal growth factor receptor antibody efficacy in BRAF-mutant metastatic colorectal cancer: a pooled analysis of AIO studies FIRE-1, CIOX, FIRE-3, XELAVIRI, and VOLFI
Abstract
Background: Primary tumor (PT) sidedness is an established prognostic marker in metastatic colorectal cancer (mCRC) and has a predictive impact on the efficacy of anti-epidermal growth factor receptor (anti-EGFR) antibody [monoclonal antibody (mAb)] in patients with RAS wild-type mCRC. This investigation focuses on patients with BRAFV600E-mutated (BRAFmt) mCRC and examines the efficacy of anti-EGFR mAbs in relation to primary tumor sidedness (PTS).
Patient and methods: This pooled analysis was carried out using individual patient data from five randomized studies in the first-line setting of mCRC. The population of interest was limited to patients with BRAFmt mCRC and known PTS. For analysis, treatment was stratified into two groups: those treated with anti-EGFR mAbs and those without. Dichotomous variables, such as overall response rate and objective response rate (ORR), were compared using chi-square or Fisher's exact test. Time-to-event endpoints [progression-free survival (PFS) and overall survival (OS)] were analyzed using the Kaplan-Meier method, log-rank test, and Cox regression. An interaction test was carried out via Cox regression.
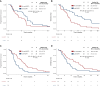
Results: A total of 102 patients with BRAFmt mCRC were identified. The type of targeted therapy (anti-EGFR-based versus non-anti-EGFR) did not significantly impact the outcome. However, in patients with left-sided primary tumors, anti-EGFR mAb-based treatment, compared with non-anti-EGFR, was associated with a higher ORR (58% versus 34%; P < 0.01), trended toward improved PFS [hazard ratio (HR) 0.62; 95% confidence interval (CI) 0.34-1.13; P = 0.12], and demonstrated prolonged OS (HR 0.38; 95% CI 0.20-0.72; P < 0.01). In patients with right-sided primary tumors, anti-EGFR-based therapy had no effect on ORR (33% versus 36%; P > 0.99), induced inferior PFS (HR 1.97; 95% CI 1.12-3.47; P = 0.02), and trended toward a worse OS (HR 1.76; 95% CI 0.99-3.13; P = 0.05).
Conclusion: This analysis suggests that PTS has predictive value for the efficacy of anti-EGFR mAb in the first-line treatment of BRAFmt mCRC.
Keywords: BRAF mutation; EGFR antibody; metastatic colorectal cancer; primary tumor location; primary tumor sidedness.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

