Twenty-Three Years of Declining Lithium Use: Analysis of a Pharmacoepidemiological Dataset from German-Speaking Countries
- PMID: 39173675
- PMCID: PMC11543241
- DOI: 10.1055/a-2374-2386
Twenty-Three Years of Declining Lithium Use: Analysis of a Pharmacoepidemiological Dataset from German-Speaking Countries
Abstract
Introduction: Pharmacoepidemiological data suggest that lithium prescriptions for bipolar disorder are gradually decreasing, with less attention having been paid to other indications.
Methods: We examined lithium prescriptions between 1994 and 2017 in data provided by the Drug Safety in Psychiatry Program AMSP, including psychiatric hospitals in Germany, Austria and Switzerland. We compared lithium use for different diagnoses before and after 2001 and in three periods (T1: 1994-2001, T2: 2002-2009, and T3: 2010-2017).
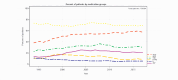
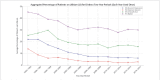
Results: In a total of 158,384 adult inpatients (54% female, mean age 47.4±17.0 years), we observed a statistically significant decrease in lithium prescriptions between 1994-2000 and 2001-2017 in patients with schizophrenia spectrum disorder from 7.7% to 5.1% and in patients with affective disorders from 16.8% to 9.6%. Decreases in use were also observed for diagnostic subgroups: schizoaffective disorder (ICD-10 F25: 27.8% to 17.4%), bipolar disorder (F31: 41.3% to 31%), depressive episode (F32: 8.1% to 3.4%), recurrent depression (F33: 17.9% to 7.5%, all: p<0.001) and emotionally unstable (borderline) personality disorder (6.3% to 3.9%, p=0.01). The results in T1 vs. T2 vs. T3 were for F25: 26.7% vs. 18.2% vs. 16.2%, F32: 7.7% vs. 4.2% vs. 2.7%, F33: 17.2% vs. 8.6% vs. 6.6% and for F31: 40.8% vs. 31.7% vs 30.0%, i. e. there was no further decrease for lithium use in bipolar disorder after 2002. Lithium's main psychotropic co-medications were quetiapine (21.1%), lorazepam (20.6%), and olanzapine (15.2%).
Discussion: In inpatients, the use of lithium has decreased in patients with bipolar disorder and also with various other psychiatric diagnoses.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
Competing interests S. Toto is the project manager of the AMSP program and a member of the advisory board for Otsuka and Janssen-Cilag and has received speaker’s honoraria from Janssen-Cilag, Lundbeck/Otsuka, Recordati Pharma GmbH, ROVI, and Servier. G. Schoretsanitis has served as a consultant for Dexcel Pharma, HLS Therapeutics, Saladax and Thermo Fisher and has received speaker’s fees from HLS Therapeutics and Saladax. M. de Bardeci is Co-Founder and member of the board of directors of the company DeepPsy AG. All other authors state they have no conflicts of interest to declare.Role of the funding sourceThe authors did not receive any specific grants or funding for the present study. The AMSP drug safety program is facilitated by nonprofit associations in Germany, Austria and Switzerland. The AMSP program has been supported with unrestricted educational and research grants since 1993 by the following companies: Austrian companies: Astra Zeneca Österreich GmbH, Boehringer Ingelheim Austria, Bristol-Myers Squibb GmbH, CSC Pharmaceuticals GmbH, Eli Lilly GmbH, Germania Pharma GmbH, GlaxoSmithKline Pharma GmbH, Janssen-Cilag Pharma GmbH, Lundbeck GmbH, Novartis Pharma GmbH, Pfizer Med Inform and Wyeth Lederle Pharma GmbH; German companies: Abbott GmbH & Co. KG, Aristo Pharma, AstraZeneca GmbH, Aventis Pharma Deutschland GmbH GE–O/R/N, Bayer Vital GmbH, Boehringer Mannheim GmbH, Bristol-Myers-Squibb, Ciba Geigy GmbH, Desitin Arzneimittel GmbH, Duphar Pharma GmbH & Co. KG, Eisai GmbH, Esparma GmbH Arzneimittel, GlaxoSmithKline Pharma GmbH & Co. KG, Hoffmann-La Roche AG Medical Affairs, Janssen-Cilag GmbH, Janssen Research Foundation, Knoll Deutschland GmbH, Lilly Deutschland GmbH Niederlassung Bad Homburg, Lundbeck GmbH & Co. KG, Novartis Pharma GmbH, Nordmark Arzneimittel GmbH, Organon GmbH, Otsuka-Pharma Frankfurt, Pfizer GmbH, Pharmacia & Upjohn GmbH, Promonta Lundbeck Arzneimittel, Recordati Pharma GmbH, Rhone-Poulenc Rohrer, ROVI, Sanofi-Synthelabo GmbH, Sanofi-Aventis Deutschland, Schering AG, SmithKlineBeecham Pharma GmbH, Solvay Arzneimittel GmbH, Synthelabo Arzneimittel GmbH, Dr. Wilmar Schwabe GmbH & Co., Thiemann Arzneimittel GmbH, Troponwerke GmbH & Co. KG, Upjohn GmbH, Wander Pharma GmbH and Wyeth-Pharma GmbH; Swiss companies: AHP (Schweiz) AG, AstraZeneca AG, Bristol-Myers Squibb AG, Desitin Pharma GmbH, Eli Lilly (Suisse) S.A., Essex Chemie AG, GlaxoSmithKline AG, Janssen-Cilag AG, Lundbeck (Suisse) AG, Organon AG, Pfizer AG, Pharmacia, Sanofi-Aventis (Suisse) S.A., Sanofi-Synthelabo SA, Servier SA, SmithKlineBeecham AG, Solvay Pharma AG, Wyeth AHP (Suisse) AG and Wyeth Pharmaceuticals AG.
Figures
References
-
- Ercis M, Ozerdem A, Singh B. When and how to use lithium augmentation for treating major depressive disorder. J Clin Psychiatry. 2023;84:23ac14813. - PubMed
-
- Antolin-Concha D, Lahteenvuo M, Vattulainen P et al. Suicide mortality and use of psychotropic drugs in patients hospitalized due to bipolar disorder: A Finnish nationwide cohort study. J Affect Disord. 2020;277:885–892. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

