Diet, Microbiome, and Inflammation Predictors of Fecal and Plasma Short-Chain Fatty Acids in Humans
- PMID: 39173973
- PMCID: PMC11600052
- DOI: 10.1016/j.tjnut.2024.08.012
Diet, Microbiome, and Inflammation Predictors of Fecal and Plasma Short-Chain Fatty Acids in Humans
Abstract
Background: Gut microbes produce short-chain fatty acids (SCFAs), which are associated with broad health benefits. However, it is not fully known how diet and/or the gut microbiome could be modulated to improve SCFA production.
Objectives: The objective of this study was to identify dietary, inflammatory, and/or microbiome predictors of SCFAs in a cohort of healthy adults.
Methods: SCFAs were measured in fecal and plasma samples from 359 healthy adults in the United States Department of Agriculture Nutritional Phenotyping Study. Habitual and recent diet was assessed using a Food Frequency Questionnaire and Automated Self-Administered 24-h Dietary Assesment Tool dietary recalls. Markers of systemic and gut inflammation were measured in fecal and plasma samples. The gut microbiome was assessed using shotgun metagenomics. Using statistics and machine learning, we determined how the abundance and composition of SCFAs varied with measures of diet, inflammation, and the gut microbiome.
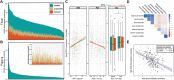
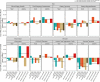
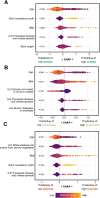
Results: We show that fecal pH may be a good proxy for fecal SCFA abundance. A higher Healthy Eating Index for a habitual diet was associated with a compositional increase in fecal butyrate relative to acetate and propionate. SCFAs were associated with markers of subclinical gastrointestinal (GI) inflammation. Fecal SCFA abundance was inversely related to plasma lipopolysaccharide-binding protein. When we analyzed hierarchically organized diet and microbiome data with taxonomy-aware algorithms, we observed that diet and microbiome features were far more predictive of fecal SCFA abundances compared to plasma SCFA abundances. The top diet and microbiome predictors of fecal butyrate included potatoes and the thiamine biosynthesis pathway, respectively.
Conclusions: These results suggest that resistant starch in the form of potatoes and microbially produced thiamine provide a substrate and essential cofactor, respectively, for butyrate synthesis. Thiamine may be a rate-limiting nutrient for butyrate production in adults. Overall, these findings illustrate the complex biology underpinning SCFA production in the gut. This trial was registered at clinicaltrials.gov as NCT02367287.
Keywords: diet; gut microbiome; inflammation; machine learning; short-chain fatty acids.
Published by Elsevier Inc.
Figures