High-Dose Propranolol for Severe and Chronic Aggression in Autism Spectrum Disorder: A Pilot, Double-Blind, Placebo-Controlled, Randomized Crossover Study
- PMID: 39174017
- PMCID: PMC11460740
- DOI: 10.1097/JCP.0000000000001895
High-Dose Propranolol for Severe and Chronic Aggression in Autism Spectrum Disorder: A Pilot, Double-Blind, Placebo-Controlled, Randomized Crossover Study
Abstract
Background: Despite the use of behavioral interventions and psychotropic medications, many individuals with autism spectrum disorder (ASD) who engage in severe aggression remain refractory to conventional treatment. Propranolol, a beta-blocker, has accumulated much anecdotal evidence as a promising option. However, well-designed studies are rare, and the apprehension about cardiovascular side effects from large doses continues to exist.
Purpose: The aims of this study were (1) to demonstrate the feasibility of treating aggression with high-dose propranolol using telehealth study visits and (2) to document cardiac safety.
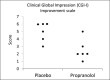
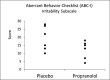
Methods: This study utilized a randomized, double-blind, placebo-controlled, crossover design. Dosing was titrated up in a flexible but stepwise fashion until therapeutic response was obtained or up to 200 mg tid. Following washout, those who were assigned propranolol were crossed over to placebo and vice versa. Six participants between the ages 12-19 participated. The primary outcome measures were the final Clinical Global Impression Improvement Scale (CGI-I) and the Aberrant Behavior Checklist-Community Irritability (ABC-C/I) scores at 200 mg tid.
Results: The CGI-I indicated a 50% reduction in symptoms in the propranolol phase, while the ABC-I indicated a 37% reduction in comparison to placebo. The effect sizes ( r ) for the CGI-I and the ABC-C/I were large, -0.74 and -0.64, respectively. The average blood pressure was 122/68 during the placebo phase and 109/72 during the propranolol phase. All Holter monitor exams were unremarkable.
Conclusion: These results suggest that propranolol is an effective option in decreasing aggression in individuals with ASD. As this was a small study, a larger clinical trial is needed.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures
References
-
- Kanne SM, Mazurek MO. Aggression in children and adolescents with ASD: prevalence and risk factors. J Autism Dev Disord. 2011;41:926–937. - PubMed
-
- Laverty C Agar G Sinclair-Burton L, et al. The 10-year trajectory of aggressive behaviours in autistic individuals. J Intellect Disabil Res. 2023;67:295–309. - PubMed
-
- Myrbakk E, von Tetzchner S. Psychiatric disorders and behavior problems in people with intellectual disability. Res Dev Disabil. 2008;29:316–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials