Protocol for a phase 3, randomised, active-control study of four-factor prothrombin complex concentrate versus frozen plasma in bleeding adult cardiac surgery patients requiring coagulation factor replacement: the LEX-211 (FARES-II) trial
- PMID: 39174056
- PMCID: PMC11344867
- DOI: 10.1136/bmjopen-2024-091381
Protocol for a phase 3, randomised, active-control study of four-factor prothrombin complex concentrate versus frozen plasma in bleeding adult cardiac surgery patients requiring coagulation factor replacement: the LEX-211 (FARES-II) trial
Abstract
Introduction: Reduced thrombin generation is an important component of post cardiopulmonary bypass (CPB) coagulopathy. To replenish coagulation factors and enhance thrombin generation in bleeding surgical patients, frozen plasma (FP) and four-factor prothrombin complex concentrate (4F-PCC) are used. However, the efficacy-safety balance of 4F-PCC relative to FP in cardiac surgery is unconfirmed.
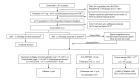
Methods and analysis: LEX-211 (FARES-II) is an active-control, randomised, phase 3 study comparing two coagulation factor replacement therapies in bleeding adult cardiac surgical patients at 12 hospitals in Canada and the USA. The primary objective is to determine whether 4F-PCC (Octaplex/Balfaxar, Octapharma) is clinically non-inferior to FP for haemostatic effectiveness. Inclusion criteria are any index (elective or non-elective) cardiac surgery employing CPB and coagulation factor replacement with 4F-PCC or FP ordered in the operating room for bleeding management. Patients will be randomised to receive 1500 or 2000 international units of 4F-PCC or 3 or 4 units of FP, depending on body weight. The primary endpoint of haemostatic treatment response is 'effective' if no additional haemostatic intervention is required from 60 min to 24 hours after the first initiation of 4F-PCC or FP; or 'ineffective' if any other haemostatic intervention (including a second dose of study drug) is required. An estimated 410 evaluable patients will be required to demonstrate non-inferiority (one-sided α of 0.025, power ≥90%, non-inferiority margin 0.10). Secondary outcomes include transfusions, bleeding-related clinical endpoints, coagulation parameters and safety.
Ethics and dissemination: The trial has been approved by the institutional review boards of all participating centres. Trial completion is anticipated at the end of 2024, and results will be disseminated via publications in peer-reviewed journals and conference presentations in 2025. The results will advance our understanding of coagulation management in bleeding surgical patients, potentially reducing the need for allogeneic blood products and improving outcomes in surgical patients.
Trial registration number: NCT05523297.
Keywords: Bleeding disorders & coagulopathies; Blood bank & transfusion medicine; Cardiac surgery; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: KK has received research funding or honoraria from Octapharma, Werfen, and Genentech. JB has received research funding or honoraria from Octapharma, Grifols, and Canadian Blood Services. JC has received research funding or honoraria from Octapharma and Canadian Blood Services. CS and SK are employees of Octapharma. JL serves on Advisory or Steering Committees for Bayer, Grifols, Octapharma, Takeda, and Werfen.
Figures

References
-
- Karkouti K, Callum J, Rao V, et al. Protocol for a phase III, non-inferiority, randomised comparison of a new fibrinogen concentrate versus cryoprecipitate for treating acquired hypofibrinogenaemia in bleeding cardiac surgical patients: the FIBRES trial. BMJ Open. 2018;8:e020741. doi: 10.1136/bmjopen-2017-020741. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
