Treating severe paediatric asthma with mepolizumab or omalizumab: a protocol for the TREAT randomised non-inferiority trial
- PMID: 39174059
- PMCID: PMC11340717
- DOI: 10.1136/bmjopen-2024-090749
Treating severe paediatric asthma with mepolizumab or omalizumab: a protocol for the TREAT randomised non-inferiority trial
Abstract
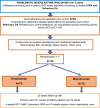
Introduction: A minority of school-aged children with asthma have persistent poor control and experience frequent asthma attacks despite maximal prescribed maintenance therapy. These children have higher morbidity and risk of death. The first add-on biologic therapy, omalizumab, a monoclonal antibody that blocks immunoglobulin (Ig)E, was licensed for children with severe asthma in 2005. While omalizumab is an effective treatment, non-response is common. A second biologic, mepolizumab which blocks interleukin 5 and targets eosinophilic inflammation, was licensed in 2018, but the licence was granted by extrapolation of adult clinical trial data to children. This non-inferiority (NI) trial will determine whether mepolizumab is as efficacious as omalizumab in reducing asthma attacks in children with severe therapy resistant asthma (STRA) and refractory difficult asthma (DA).
Methods and analysis: This is an ongoing multicentre 1:1 randomised NI open-label trial of mepolizumab and omalizumab. Up to 150 children and young people (CYP) aged 6-17 years with severe asthma will be recruited from specialist paediatric severe asthma centres in the UK. Prior to randomisation, children will be monitored for medication adherence for up to 16 weeks to determine STRA and refractory DA diagnoses. Current prescribing recommendations of serum IgE and blood eosinophils will not influence eligibility or enrolment. The primary outcome is the 52-week asthma attack rate. Bayesian analysis using clinician-elicited prior distributions will be used to calculate the posterior probability that mepolizumab is not inferior to omalizumab. Secondary outcomes include Composite Asthma Severity Index, Paediatric Asthma Quality of Life Questionnaire, lung function measures (forced expiratory volume in one second (FEV1), bronchodilator reversibility), fractional exhaled nitric oxide, Asthma Control Test (ACT), health outcomes EuroQol 5 Dimension (EQ-5D) and optimal serum IgE and blood eosinophil levels that may predict a response to therapy. These outcomes will be analysed in a frequentist framework using longitudinal models.
Ethics and dissemination: The study has been approved by the South Central-Berkshire Research Ethics Committee REC Number 19/SC/0634 and had Clinical Trials Authorisation from the Medicines and Healthcare Products Regulatory Agency (MHRA) (EudraCT 2019-004085-17). All parents/legal guardians will give informed consent for their child to participate in the trial, and CYP will give assent to participate. The results will be published in peer-reviewed journals, presented at international conferences and disseminated via our patient and public involvement partners.
Trial registration number: ISRCTN12109108; EudraCT Number: 2019-004085-17.
Keywords: Asthma; Clinical trials; Paediatric thoracic medicine; Randomized Controlled Trial; Respiratory Function Test.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
