Pharmacological and pre-clinical safety profile of rSIV.F/HN, a hybrid lentiviral vector for cystic fibrosis gene therapy
- PMID: 39174284
- PMCID: PMC11780724
- DOI: 10.1183/13993003.01683-2023
Pharmacological and pre-clinical safety profile of rSIV.F/HN, a hybrid lentiviral vector for cystic fibrosis gene therapy
Abstract
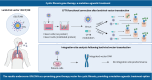
Rationale and objective: Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. CFTR modulators offer significant improvements, but ∼10% of patients remain nonresponsive or are intolerant. This study provides an analysis of rSIV.F/HN, a lentiviral vector optimised for lung delivery, including CFTR protein expression, functional correction of CFTR defects and genomic integration site analysis in preparation for a first-in-human clinical trial.
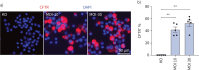
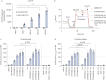
Methods: Air-liquid interface cultures of primary human bronchial epithelial cells (HBECs) from CF patients (F508del/F508del), as well as a CFTR-deficient immortalised human lung epithelial cell line mimicking class I (CFTR-null) homozygous mutations, were used to assess transduction efficiency. Quantification methods included a novel proximity ligation assay for CFTR protein expression. For assessment of CFTR channel activity, Ussing chamber studies were conducted. The safety profile was assessed using integration site analysis and in vitro insertional mutagenesis studies.
Results: rSIV.F/HN expressed CFTR and restored CFTR-mediated chloride currents to physiological levels in primary F508del/F508del HBECs as well as in a class I cells. In contrast, the latter could not be achieved by small-molecule CFTR modulators, underscoring the potential of gene therapy for this mutation class. Combination of rSIV.F/HN-CFTR with the potentiator ivacaftor showed a greater than additive effect. The genomic integration pattern showed no site predominance (frequency of occurrence ≤10%), and a low risk of insertional mutagenesis was observed in an in vitro immortalisation assay.
Conclusions: The results underscore rSIV.F/HN as a promising gene therapy vector for CF, providing a mutation-agnostic treatment option.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: A. Moiseenko reports support for the present study from Boehringer Ingelheim and UK Cystic Fibrosis Gene Therapy Consortium (UKCFGTC), and patents planned, issued or pending (02-0580-GB-1). A. Sinadinos reports support for the present study from Boehringer Ingelheim International GmbH. A. Saleh reports support for the present study from Boehringer Ingelheim International GmbH. K. Nekola reports support for the present study from Boehringer Ingelheim Pharma GmbH & Co. KG. N. Strang reports support for the present study from Boehringer Ingelheim. A.C. Boyd reports support for the present study from Boehringer Ingelheim International GmbH. J.C. Davies reports support for the present study from ArticulateScience LLC, grants from UK Cystic Fibrosis Trust, Cystic Fibrosis Foundation, Cystic Fibrosis Ireland and EPSRC, payment or honoraria for lectures, presentations, manuscript writing or educational events from Vertex Pharmaceuticals, Boehringer Ingelheim, Eloxx, Algipharma, Abbvie, Arcturus, Enterprise Therapeutics, Recode, LifeArc, and Genentech, and a leadership role with Journal of Cystic Fibrosis (Deputy Editor). D.R. Gill reports support for the present study from Boehringer Ingelheim International GmbH, and patents planned, issued or pending (lentiviral gene therapy for CF) with Boehringer Ingelheim International GmbH. S.C. Hyde reports support for the present study from Boehringer Ingelheim International GmbH, and patents planned, issued or pending (lentiviral gene therapy for CF) with Boehringer Ingelheim International GmbH. G. McLachlan reports support for the present study from Boehringer Ingelheim International GmbH. T. Rath reports support for the present study from ProtaGene CGT GmbH (former name GeneWerk GmbH). M. Schuler reports support for the present study from Boehringer Ingelheim Pharma GmbH & Co. KG. U. Maier reports support for the present study from Boehringer Ingelheim Pharma GmbH & Co. KG. D. Mennerich reports support for the present study from Boehringer Ingelheim. U. Griesenbach reports support for the present study from Boehringer Ingelheim International GmbH, patents planned, issued or pending (lentiviral gene therapy for CF) with Boehringer Ingelheim International GmbH, and leadership roles with Cell and Gene Therapy Catapult (nonexecutive director) and AlveoGene (director). E.W.F.W. Alton reports support for the present study from Boehringer Ingelheim International GmbH. S. Kreuz reports support for the present study from Boehringer Ingelheim and UK Cystic Fibrosis Gene Therapy Consortium (UKCFGTC), and patents planned, issued or pending (02-0580-GB-1). The remaining authors have no potential conflicts of interest to disclose.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
