A phase 3 study (PATHWAY) of palbociclib plus tamoxifen in patients with HR-positive/HER2-negative advanced breast cancer
- PMID: 39174547
- PMCID: PMC11341958
- DOI: 10.1038/s41523-024-00684-w
A phase 3 study (PATHWAY) of palbociclib plus tamoxifen in patients with HR-positive/HER2-negative advanced breast cancer
Abstract
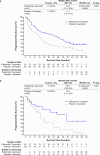
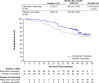
Palbociclib combined with endocrine therapy is approved for treating patients with hormone-receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced breast cancer; however, data on palbociclib combined with tamoxifen are limited. We investigated the efficacy and safety of palbociclib-tamoxifen in patients with HR+/HER2- advanced breast cancer. This double-blind phase 3 study included 184 women who were randomly assigned 1:1 to receive palbociclib-tamoxifen or placebo-tamoxifen. Pre/perimenopausal women also received goserelin. The primary endpoint was investigator-assessed progression-free survival (PFS). Secondary endpoints included overall survival (OS) and safety. Median PFS was 24.4 months (95% confidence interval [CI], 13.1-32.4) with palbociclib-tamoxifen and 11.1 months (95% CI, 7.4-14.6) with placebo-tamoxifen (hazard ratio [HR], 0.60; 95% CI, 0.43-0.85; P = 0.002). Palbociclib-tamoxifen improved PFS in patients who were treated with first-line or second-line endocrine therapy and pre-, peri-, and postmenopausal patients. Though OS data are still immature (median not reached in both groups), an overall risk reduction of 27% (HR, 0.73; 95% CI, 0.44-1.21) with palbociclib-tamoxifen was observed at the time of PFS analysis. The most common grade 3/4 adverse event with palbociclib-tamoxifen was neutropenia (89.0% [none were febrile] versus 1.1% with placebo-tamoxifen). There were no deaths owing to adverse events in either group. Among patients with HR+/HER2- advanced breast cancer, palbociclib-tamoxifen resulted in significantly longer PFS than tamoxifen alone. Early OS data showed a trend favoring palbociclib-tamoxifen. Trial registration: ClinicalTrials.gov number, NCT03423199. Study registration date: February 06, 2018.
© 2024. The Author(s).
Conflict of interest statement
Authors declare no competing non-financial interests but the following competing financial interests: Emi Noguchi: speaker honoraria from AstraZeneca, Pfizer, Chugai Pharma, Novartis. Takashi Yamanaka: honoraria from AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Lilly, Kyowa Kirin, Novartis, Pfizer; consultancy/advisory fees from Daiichi Sankyo. Hirofumi Mukai: honoraria or lecture fee from Takeda and Taiho Pharmaceutical. Naohito Yamamoto: research funding from AstraZeneca and Pfizer. Chi-Feng Chung: advisory role/speaker honoraria: AstraZeneca, Daiichi Sankyo, Pfizer, Roche, and Novartis. Yen-Shen Lu: grant support/research collaborations: Novartis, Pfizer, MSD, Roche, AstraZeneca, ACT Genomics, Advisory board/speaker invitation: Novartis, Pfizer, MSD, Roche, AstraZeneca, Eisai, Eli Lily, Daiichi Sankyo and EuroPharma, Conference support: Novartis, Eisai, and MSD. Dwan-Ying Chang: advisory role/speaker honoraria: Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, MSD, Novartis, ONO pharma, Pierre-Faber, Pfizer, Roche, Sanofi, TTY Biopharm, and EuroPharma, Conference support: Pfizer, Roche, AstraZeneca, Travel accommodation: Pfizer. Joohyuk Sohn: research funding from MSD, Roche, Novartis, Lilly, Pfizer, Daiichi Sankyo, AstraZeneca, GSK, Sanofi, Boehringer Ingelheim, and Seagen. Kyung-Hun Lee: honoraria or lecture fees from AstraZeneca, Eli Lilly, Novartis, Pfizer, and Everest Medicine. Soo-Chin Lee: honoraria from Pfizer, Novartis, AstraZeneca, ACT Genomics, Lilly, MSD, Roche, Gilead Sciences, Daiichi Sankyo, DKSH; consulting/advisory fees from Pfizer, Novartis, AstraZeneca, MSD, Roche, Gilead Sciences, Daiichi Sankyo; speakers’ fees from Pfizer, Novartis, AstraZeneca, Roche, and MSD; research funding from Pfizer, Eisai, Taiho Pharmaceutical, ACT Genomics, and Karyopharm Therapeutics; travel/accommodation expenses from Amgen, Pfizer, and Roche. Hiroji Iwata: honoraria and research funding from AstraZeneca K.K. and Pfizer, fees for promotional materials from AstraZeneca. Kenichi Watanabe: speaker’s honoraria from Chugai, Eli Lilly, Nippon- Kayaku, Kyowa Kirin, Novartis, Taiho, Eisai, Pfizer, Shionogi, Daiichi Sankyo and AstraZeneca. Kyung Hae Jung: has consulting or advisory role for AstraZeneca, Bixink, Daiichi Sankyo, Eisai, Everest Medicine, MSD, Novartis, Pfizer, Roche, and Takeda Pharmaceuticals. Yuko Tanabe: research funding from MSD. Eriko Tokunaga: personal fees for lectures from Eli Lilly, AstraZeneca, and Daiichi Sankyo. Yoon Sim Yap: Honoraria: Novartis, Pfizer, Lilly/DKSH, AstraZeneca, Eisai, MSD, Specialised Therapeutics, Roche; Research funding: Merck Sharp & Dohme; Travel, accommodations, expenses: DKSH, AstraZeneca. Koji Matsumoto: has received research funding from MSD, Chugai, Daiichi-Sankyo, Eisai, Eli Lilly, and Gilead Sciences; has received honoraria from MSD, Kyowa Kirin, and Chugai. Yoshiko Umeyama: is an employee of Pfizer R&D Japan and owns stock in Pfizer. Kazuki Sudo: honoraria from AstraZeneca, Pfizer, Eisai, Nihon Medi-Physics Co.; research funding from NanoCarrier, Daiichi Sankyo, AstraZeneca, Pfizer, Amgen, PRA Health Sciences, Takeda, and Merck. Aya Kuchiba: honoraria from Chugai Pharma. Kenichi Nakamura: honoraria from Chugai Pharma, Taiho Pharmaceutical, IQVIA, AstraZeneca, Lilly; research funding from Astellas Pharma, Eisai, Otsuka, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Takeda, Chugai/Roche, Novartis, Pfizer, Bristol-Myers Squibb Japan, Boehringer Ingelheim Seiyaku, SymBio Pharmaceuticals, Merck, and Servier. Yasuhiro Fujiwara, is now Chief Executive of Pharmaceuticals and Medical Devices Agency, regulatory agency of Japan, and contributed to this trial until March 2019. Kan Yonemori: consulting/advisory fees: Chugai Pharma, Ono Pharmaceutical, Novartis, Eisai, and OncXerna Therapeutics; honoraria from Eisai, Pfizer, AstraZeneca, Novartis, Taiho Pharmaceutical, Lilly Japan, and Daiichi Sankyo/AstraZeneca. Gun Min Kim, Tsutomu Iwasa, Seok Yun Kang, Hiroyuki Yasojima, Kenjiro Aogi, Sung Hoon Sim, Ling-Ming Tseng, Yuki Kojima, Tomomi Hata, Taro Shibata, and Kenji Tamura: no competing financial or non-financial interests.
Figures



References
-
- Thurlimann, B. et al. Anastrozole (‘Arimidex’) versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95-a sub-study of the TARGET (Tamoxifen or ‘Arimidex’ Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res. Treat.85, 247–254 (2004). 10.1023/B:BREA.0000025420.78346.f9 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

