Alveolar epithelial cells mitigate neutrophilic inflammation in lung injury through regulating mitochondrial fatty acid oxidation
- PMID: 39174557
- PMCID: PMC11341863
- DOI: 10.1038/s41467-024-51683-1
Alveolar epithelial cells mitigate neutrophilic inflammation in lung injury through regulating mitochondrial fatty acid oxidation
Abstract
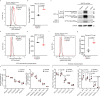
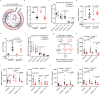
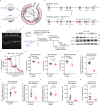
Type 2 alveolar epithelial (AT2) cells of the lung are fundamental in regulating alveolar inflammation in response to injury. Impaired mitochondrial long-chain fatty acid β-oxidation (mtLCFAO) in AT2 cells is assumed to aggravate alveolar inflammation in acute lung injury (ALI), yet the importance of mtLCFAO to AT2 cell function needs to be defined. Here we show that expression of carnitine palmitoyltransferase 1a (CPT1a), a mtLCFAO rate limiting enzyme, in AT2 cells is significantly decreased in acute respiratory distress syndrome (ARDS). In mice, Cpt1a deletion in AT2 cells impairs mtLCFAO without reducing ATP production and alters surfactant phospholipid abundance in the alveoli. Impairing mtLCFAO in AT2 cells via deleting either Cpt1a or Acadl (acyl-CoA dehydrogenase long chain) restricts alveolar inflammation in ALI by hindering the production of the neutrophilic chemokine CXCL2 from AT2 cells. This study thus highlights mtLCFAO as immunometabolism to injury in AT2 cells and suggests impaired mtLCFAO in AT2 cells as an anti-inflammatory response in ARDS.
© 2024. The Author(s).
Conflict of interest statement
A.M.K.C. is a cofounder and equity stockholder for Proterris, which develops therapeutic uses for CO. A.M.K.C. has a use patent on CO. Additionally, A.M.K.C. has patents in chronic obstructive pulmonary disease (US Patent 10905682). M.P. participates an advisory board meeting for InflaRx. All the other authors declare no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

