The ALS-associated KIF5A P986L variant is not pathogenic for Drosophila motoneurons
- PMID: 39174694
- PMCID: PMC11341546
- DOI: 10.1038/s41598-024-70543-y
The ALS-associated KIF5A P986L variant is not pathogenic for Drosophila motoneurons
Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating paralytic disorder caused by the death of motoneurons. Several mutations in the KIF5A gene have been identified in patients with ALS. Some mutations affect the splicing sites of exon 27 leading to its deletion (Δ27 mutation). KIF5A Δ27 is aggregation-prone and pathogenic for motoneurons due to a toxic gain of function. Another mutation found to be enriched in ALS patients is a proline/leucine substitution at position 986 (P986L mutation). Bioinformatic analyses strongly suggest that this variant is benign. Our study aims to conduct functional studies in Drosophila to classify the KIF5A P986L variant. When expressed in motoneurons, KIF5A P986L does not modify the morphology of larval NMJ or the synaptic transmission. In addition, KIF5A P986L is uniformly distributed in axons and does not disturb mitochondria distribution. Locomotion at larval and adult stages is not affected by KIF5A P986L. Finally, both KIF5A WT and P986L expression in adult motoneurons extend median lifespan compared to control flies. Altogether, our data show that the KIF5A P986L variant is not pathogenic for motoneurons and may represent a hypomorphic allele, although it is not causative for ALS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
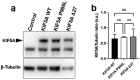
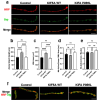
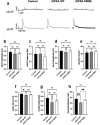


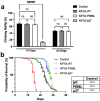
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

