Intranasal delivery of small extracellular vesicles reduces the progress of amyotrophic lateral sclerosis and the overactivation of complement-coagulation cascade and NF-ĸB signaling in SOD1G93A mice
- PMID: 39174972
- PMCID: PMC11340036
- DOI: 10.1186/s12951-024-02764-2
Intranasal delivery of small extracellular vesicles reduces the progress of amyotrophic lateral sclerosis and the overactivation of complement-coagulation cascade and NF-ĸB signaling in SOD1G93A mice
Abstract
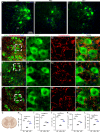
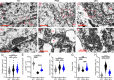
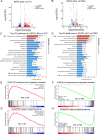
Amyotrophic lateral sclerosis (ALS) is a fatal disease characterized by progressive motoneuron degeneration, and effective clinical treatments are lacking. In this study, we evaluated whether intranasal delivery of mesenchymal stem cell-derived small extracellular vesicles (sEVs) is a strategy for ALS therapy using SOD1G93A mice. In vivo tracing showed that intranasally-delivered sEVs entered the central nervous system and were extensively taken up by spinal neurons and some microglia. SOD1G93A mice that intranasally received sEV administration showed significant improvements in motor performances and survival time. After sEV administration, pathological changes, including spinal motoneuron death and synaptic denervation, axon demyelination, neuromuscular junction degeneration and electrophysiological defects, and mitochondrial vacuolization were remarkably alleviated. sEV administration attenuated the elevation of proinflammatory cytokines and glial responses. Proteomics and transcriptomics analysis revealed upregulation of the complement and coagulation cascade and NF-ĸB signaling pathway in SOD1G93A mouse spinal cords, which was significantly inhibited by sEV administration. The changes were further confirmed by detecting C1q and NF-ĸB expression using Western blots. In conclusion, intranasal administration of sEVs effectively delays the progression of ALS by inhibiting neuroinflammation and overactivation of the complement and coagulation cascades and NF-ĸB signaling pathway and is a potential option for ALS therapy.
Keywords: Amyotrophic lateral sclerosis; Complement & coagulation cascade; Intranasal administration; Motoneuron degeneration; NF-ĸB signaling; Neuroinflammation; Proteomics; Small extracellular vesicles; Transcriptomics; Transgenic mouse.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

