Inhibition of forward and reverse transport of Ca2+ via Na+/Ca2+ exchangers (NCX) prevents sperm capacitation
- PMID: 39175101
- PMCID: PMC11342557
- DOI: 10.1186/s40659-024-00535-9
Inhibition of forward and reverse transport of Ca2+ via Na+/Ca2+ exchangers (NCX) prevents sperm capacitation
Abstract
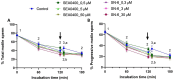
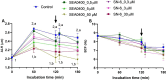
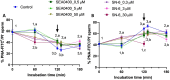
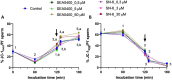
Background: While calcium is known to play a crucial role in mammalian sperm physiology, how it flows in and out of the male gamete is not completely understood. Herein, we investigated the involvement of Na+/Ca2+ exchangers (NCX) in mammalian sperm capacitation. Using the pig as an animal model, we first confirmed the presence of NCX1 and NCX2 isoforms in the sperm midpiece. Next, we partially or totally blocked Ca2+ outflux (forward transport) via NCX1/NCX2 with different concentrations of SEA0400 (2-[4-[(2,5-difluorophenyl)methoxy]phenoxy]-5-ethoxyaniline; 0, 0.5, 5 and 50 µM) and Ca2+ influx (reverse transport) with SN6 (ethyl 2-[[4-[(4-nitrophenyl)methoxy]phenyl]methyl]-1,3-thiazolidine-4-carboxylate; 0, 0.3, 3 or 30 µM). Sperm were incubated under capacitating conditions for 180 min; after 120 min, progesterone was added to induce the acrosome reaction. At 0, 60, 120, 130, and 180 min, sperm motility, membrane lipid disorder, acrosome integrity, mitochondrial membrane potential (MMP), tyrosine phosphorylation of sperm proteins, and intracellular levels of Ca2+, reactive oxygen species (ROS) and superoxides were evaluated.
Results: Partial and complete blockage of Ca2+ outflux and influx via NCX induced a significant reduction of sperm motility after progesterone addition. Early alterations on sperm kinematics were also observed, the effects being more obvious in totally blocked than in partially blocked samples. Decreased sperm motility and kinematics were related to both defective tyrosine phosphorylation and mitochondrial activity, the latter being associated to diminished MMP and ROS levels. As NCX blockage did not affect the lipid disorder of plasma membrane, the impaired acrosome integrity could result from reduced tyrosine phosphorylation.
Conclusions: Inhibition of outflux and influx of Ca2+ triggered similar effects, thus indicating that both forward and reverse Ca2+ transport through NCX exchangers are essential for sperm capacitation.
Keywords: In vitro capacitation and Ca2+ transport; Na+/Ca2+ exchangers (NCXs); Sperm.
© 2024. The Author(s).
Conflict of interest statement
Not applicable.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

