Assessment of the potential risk of oteseconazole and two other tetrazole antifungals to inhibit adrenal steroidogenesis and peripheral metabolism of corticosteroids
- PMID: 39175536
- PMCID: PMC11338861
- DOI: 10.3389/fphar.2024.1394846
Assessment of the potential risk of oteseconazole and two other tetrazole antifungals to inhibit adrenal steroidogenesis and peripheral metabolism of corticosteroids
Abstract
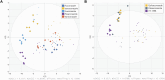
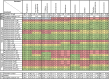
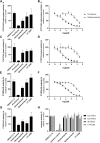
The triazole antifungals posaconazole and itraconazole can cause pseudohyperaldosteronism with hypertension and hypokalemia, edema, and gynecomastia by inhibiting steroid synthesis and metabolism. Mechanisms underlying pseudohyperaldosteronism include inhibition of adrenal 11β-hydroxylase cytochrome-P450 (CYP) 11B1 and 17α-hydroxylase (CYP17A1) as well as peripherally expressed 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). To enhance specificity for fungal CYP51, tetrazoles have been developed. This study employed H295R adrenocortical cells and enzyme activity assays to assess the potential risk of oteseconazole and two other tetrazoles, VT-1598 and quilseconazole, to inhibit adrenal steroidogenesis or 11β-HSD2. Steroidomic footprint analyses of H295R cell supernatants using untargeted liquid-chromatography-high-resolution mass-spectrometry (LC-HRMS) indicated overall patterns common to oteseconazole, quilseconazole and itraconazole, as well as similarities between VT-1598 and isavuconazole. Additionally, more specific features of the steroid signatures were observed. Targeted quantification of nine adrenal steroids in supernatants from treated H295R cells revealed an overall inhibition of adrenal steroidogenesis by the three tetrazoles, itraconazole and isavuconazole, providing an explanation for their similar steroidomic pattern. Applying recombinant enzymes indicated that this effect is not due to direct inhibition of steroidogenic enzymes because no or only weak inhibition could be observed. Moreover, oteseconazole and the two other tetrazoles did not inhibit 11β-HSD2, suggesting that they do not pose a risk of pseudohyperaldosteronism. Furthermore, oteseconazole did not alter steroid concentrations in a recent clinical study. Nevertheless, follow-up studies should assess the mechanism underlying the observed overall steroidogenesis inhibition by tetrazoles, itraconazole and isavuconazole, and whether concentrations achievable in a subgroup of susceptible patients might cause adrenal insufficiency and hyperplasia.
Keywords: H295R; adverse drug reaction; azole antifungal; cytochrome P450; enzyme; inhibition; steroid profile; steroidogenesis.
Copyright © 2024 Jäger, González-Ruiz, Joos, Winter, Boccard, Degenhardt, Brand, Rudaz, Thompson and Odermatt.
Conflict of interest statement
Authors TD and SB were employed by the Mycovia Pharmaceuticals Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Characterization of the interferences of systemic azole antifungal drugs with adrenal steroid biosynthesis using H295R cells and enzyme activity assays.Curr Res Toxicol. 2023 Aug 14;5:100119. doi: 10.1016/j.crtox.2023.100119. eCollection 2023. Curr Res Toxicol. 2023. PMID: 37637492 Free PMC article.
-
Molecular mechanisms of posaconazole- and itraconazole-induced pseudohyperaldosteronism and assessment of other systemically used azole antifungals.J Steroid Biochem Mol Biol. 2020 May;199:105605. doi: 10.1016/j.jsbmb.2020.105605. Epub 2020 Jan 23. J Steroid Biochem Mol Biol. 2020. PMID: 31982514
-
Species-specific differences in the inhibition of 11β-hydroxysteroid dehydrogenase 2 by itraconazole and posaconazole.Toxicol Appl Pharmacol. 2021 Feb 1;412:115387. doi: 10.1016/j.taap.2020.115387. Epub 2020 Dec 31. Toxicol Appl Pharmacol. 2021. PMID: 33387577
-
Steroidogenesis in guinea pig adrenal cortex: effects of ACTH on steroid secretion and steroidogenic enzyme activities and expression.J Steroid Biochem Mol Biol. 1992 Dec;43(8):855-62. doi: 10.1016/0960-0760(92)90312-7. J Steroid Biochem Mol Biol. 1992. PMID: 22217829 Review.
-
Classic and current concepts in adrenal steroidogenesis: a reappraisal.Arch Endocrinol Metab. 2022 Mar 8;66(1):77-87. doi: 10.20945/2359-3997000000438. Arch Endocrinol Metab. 2022. PMID: 35263051 Free PMC article. Review.
References
-
- Akram M., Patt M., Kaserer T., Temml V., Waratchareeyakul W., Kratschmar D. V., et al. (2019). Identification of the fungicide epoxiconazole by virtual screening and biological assessment as inhibitor of human 11β-hydroxylase and aldosterone synthase. J. steroid Biochem. Mol. Biol. 192, 105358. 10.1016/j.jsbmb.2019.04.007 - DOI - PubMed

