Therapeutic targeting of PARP with immunotherapy in acute myeloid leukemia
- PMID: 39175540
- PMCID: PMC11338796
- DOI: 10.3389/fphar.2024.1421816
Therapeutic targeting of PARP with immunotherapy in acute myeloid leukemia
Abstract
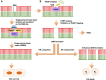
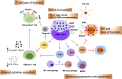
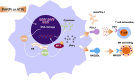
Targeting the poly (ADP-ribose) polymerase (PARP) protein has shown therapeutic efficacy in cancers with homologous recombination (HR) deficiency due to BRCA mutations. Only small fraction of acute myeloid leukemia (AML) cells carry BRCA mutations, hence the antitumor efficacy of PARP inhibitors (PARPi) against this malignancy is predicted to be limited; however, recent preclinical studies have demonstrated that PARPi monotherapy has modest efficacy in AML, while in combination with cytotoxic chemotherapy it has remarkable synergistic antitumor effects. Immunotherapy has revolutionized therapeutics in cancer treatment, and PARPi creates an ideal microenvironment for combination therapy with immunomodulatory agents by promoting tumor mutation burden. In this review, we summarize the role of PARP proteins in DNA damage response (DDR) pathways, and discuss recent preclinical studies using synthetic lethal modalities to treat AML. We also review the immunomodulatory effects of PARPi in AML preclinical models and propose future directions for therapy in AML, including combined targeting of the DDR and tumor immune microenvironment; such combination regimens will likely benefit patients with AML undergoing PARPi-mediated cancer therapy.
Keywords: AML; DNA repair; PARP; immuotherapy; synergisctic effects.
Copyright © 2024 Bian, Liu, Yang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Targeting PARP proteins in acute leukemia: DNA damage response inhibition and therapeutic strategies.J Hematol Oncol. 2022 Jan 22;15(1):10. doi: 10.1186/s13045-022-01228-0. J Hematol Oncol. 2022. PMID: 35065680 Free PMC article. Review.
-
Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: the rationale for their inclusion in the clinic.J Exp Clin Cancer Res. 2016 Nov 24;35(1):179. doi: 10.1186/s13046-016-0456-2. J Exp Clin Cancer Res. 2016. PMID: 27884198 Free PMC article. Review.
-
PARP Inhibitors and Myeloid Neoplasms: A Double-Edged Sword.Cancers (Basel). 2021 Dec 20;13(24):6385. doi: 10.3390/cancers13246385. Cancers (Basel). 2021. PMID: 34945003 Free PMC article. Review.
-
PARP goes the weasel! Emerging role of PARP inhibitors in acute leukemias.Blood Rev. 2021 Jan;45:100696. doi: 10.1016/j.blre.2020.100696. Epub 2020 May 7. Blood Rev. 2021. PMID: 32482307 Free PMC article. Review.
-
Targeting ADP-ribosylation by PARP inhibitors in acute myeloid leukaemia and related disorders.Biochem Pharmacol. 2019 Sep;167:133-148. doi: 10.1016/j.bcp.2019.04.019. Epub 2019 Apr 24. Biochem Pharmacol. 2019. PMID: 31028744 Review.
References
-
- Al-Matary Y. S., Botezatu L., Opalka B., Hones J. M., Lams R. F., Thivakaran A., et al. (2016). Acute myeloid leukemia cells polarize macrophages towards a leukemia supporting state in a Growth factor independence 1 dependent manner. Haematologica 101, 1216–1227. 10.3324/haematol.2016.143180 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

