Pilot use of a mycolactone-specific lateral flow assay for Buruli ulcer: A case report from Japan
- PMID: 39175914
- PMCID: PMC11338991
- DOI: 10.1016/j.jctube.2024.100469
Pilot use of a mycolactone-specific lateral flow assay for Buruli ulcer: A case report from Japan
Abstract
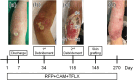
Buruli ulcer, caused by Mycobacterium (M.) ulcerans, is a neglected tropical disease (NTD) characterized by necrosis of the cutaneous tissue, predominantly affecting the limbs. The pathogenesis of this disease is mainly attributed to mycolactone, a lipid toxin produced by M. ulcerans. Here, we report the case of a 7-year-old Japanese girl who presented with worsening ulceration on her left forearm, extending to the elbow, following antimicrobial treatment. To evaluate disease progression, we used a mycolactone-specific lateral flow assay. The test yielded positive results in the advancing necrotic area, aiding in determining the extent of necessary debridement. After undergoing two debridement surgeries and receiving 38 weeks of antimicrobial treatment followed by skin grafting, the patient achieved cure. Timely diagnosis is imperative in avoiding prolonged treatment, highlighting the importance of readily available diagnostic point-of-care tests for Buruli ulcer. Moreover, detection of mycolactone not only can serve as a diagnostic tool for Buruli ulcer but also enables prediction of lesion spread and assessment of cure.
Keywords: Japan; Mycobacterium ulcerans; Paradoxical reaction; Pediatrics; Point-of-care test; Rapid diagnostic test.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dziedzom K. de Souza, Marco Biamonte, Gerd Pluschke, Rie R. Yotsu reports financial support was provided by Global Health Innovative Technology (GHIT) Fund (G2020-202). Yuji Miyamoto, Manabu Ato reports financial support was provided by Japan Agency for Medical Research and Development. Rie Yotsu is one of the guest editors for the Special Issue on Buruli Ulcer in the Journal of Clinical Tuberculosis and Other Mycobacterial Diseases. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

