Deciphering the Genetic Landscape: Insights Into the Genomic Signatures of Changle Goose
- PMID: 39175938
- PMCID: PMC11340016
- DOI: 10.1111/eva.13768
Deciphering the Genetic Landscape: Insights Into the Genomic Signatures of Changle Goose
Abstract
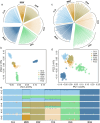
The Changle goose (CLG), a Chinese indigenous breed, is celebrated for its adaptability, rapid growth, and premium meat quality. Despite its agricultural value, the exploration of its genomic attributes has been scant. Our study entailed whole-genome resequencing of 303 geese across CLG and five other Chinese breeds, revealing distinct genetic diversity metrics. We discovered significant migration events from Xingguo gray goose to CLG and minor gene flow between them. We identified genomic regions through selective sweep analysis, correlating with CLG's unique traits. An elevated inbreeding coefficient in CLG, alongside reduced heterozygosity and rare single nucleotide polymorphisms (RSNPs), suggests a narrowed genetic diversity. Genomic regions related to reproduction, meat quality, and growth were identified, with the GATA3 gene showing strong selection signals for meat quality. A non-synonymous mutation in the Sloc2a1 gene, which is associated with reproductive traits in the CLG, exhibited significant differences in allelic frequency. The roles of CD82, CDH8, and PRKAB1 in growth and development, alongside FABP4, FAF1, ESR1, and AKAP12 in reproduction, were highlighted. Additionally, Cdkal1 and Mfsd14a may influence meat quality. This comprehensive genetic analysis underpins the unique genetic makeup of CLG, providing a basis for its conservation and informed breeding strategies.
Keywords: Changle goose; conservation genetics; genetic diversity; genomic analysis; meat quality; reproduction.
© 2024 The Author(s). Evolutionary Applications published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Anderson, F. , Pannier L., Pethick D. W., and Gardner G. E.. 2015. “Intramuscular fat in Lamb Muscle and the Impact of Selection for Improved Carcass Lean Meat Yield.” Animal 9, no. 6: 1081–1090. - PubMed
-
- Bergsma, A. , Ganguly S. S., Dick D., Williams B. O., and Miranti C. K.. 2018. “Global Deletion of Tetraspanin CD82 Attenuates Bone Growth and Enhances Bone Marrow Adipogenesis.” Bone 113: 105–113. - PubMed
-
- Bruford, M. W. 2004. “Conservation Genetics of UK Livestock: From Molecules to Management.” BSAP Occasional Publication 30: 151–169.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

