Astrocyte-derived exosomal miR-378a-5p mitigates cerebral ischemic neuroinflammation by modulating NLRP3-mediated pyroptosis
- PMID: 39176087
- PMCID: PMC11338813
- DOI: 10.3389/fimmu.2024.1454116
Astrocyte-derived exosomal miR-378a-5p mitigates cerebral ischemic neuroinflammation by modulating NLRP3-mediated pyroptosis
Abstract
Objective: This study aimed to investigate the regulatory role of astrocyte-derived exosomes and their microRNAs (miRNAs) in modulating neuronal pyroptosis during cerebral ischemia.
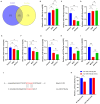
Methods: Astrocyte-derived exosomes were studied for treating cerebral ischemia in both in vitro and in vivo models. The effects of astrocyte-derived exosomes on neuroinflammation were investigated by analyzing exosome uptake, nerve damage, and pyroptosis protein expression. High throughput sequencing was used to identify astrocyte-derived exosomal miRNAs linked to pyroptosis, followed by validation via qRT‒PCR. The relationship between these miRNAs and NLRP3 was studied using a dual luciferase reporter assay. This study used miR-378a-5p overexpression and knockdown to manipulate OGD injury in nerve cells. The impact of astrocyte-derived exosomal miR-378a-5p on the regulation of cerebral ischemic neuroinflammation was assessed through analysis of nerve injury and pyroptosis protein expression.
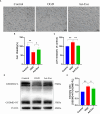
Results: Our findings demonstrated that astrocyte-derived exosomes were internalized by neurons both in vitro and in vivo. Additionally, Astrocyte-derived exosomes displayed a neuroprotective effect against OGD-induced neuronal injury and brain injury in the ischemic cortical region of middle cerebral artery occlusion (MCAO) rats while also reducing pyroptosis. Further investigations revealed the involvement of astrocyte-derived exosomal miR-378a-5p in regulating pyroptosis by inhibiting NLRP3. The overexpression of miR-378a-5p mitigated neuronal damage, whereas the knockdown of miR-378a-5p increased NLRP3 expression and exacerbated pyroptosis, thus reversing this neuroprotective effect.
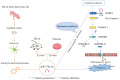
Conclusion: Astrocyte-derived exosomal miR-378a-5p has a neuroprotective effect on cerebral ischemia by suppressing neuroinflammation associated with NLRP3-mediated pyroptosis.Further research is required to comprehensively elucidate the signaling pathways by which astrocyte-derived exosomal miR-378a-5p modulates neuronal pyroptosis.
Keywords: NLRP3; astrocyte-derived exosomes; cerebral ischemia; inflammation; miR-378a-5p; pyroptosis.
Copyright © 2024 Sun, Liao, Lang, Qin, Jiao, Shao, Deng and She.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Glycosides of Buyang Huanwu decoction alleviate cerebral infarction injury by inhibiting neuronal pyroptosis via astrocyte-derived exosomal miR-378b_L+1.J Ethnopharmacol. 2025 Aug 29;352:120234. doi: 10.1016/j.jep.2025.120234. Epub 2025 Jul 3. J Ethnopharmacol. 2025. PMID: 40617362
-
miR-155-5p accelerates cerebral ischemia-reperfusion inflammation injury and cell pyroptosis via DUSP14/ TXNIP/NLRP3 pathway.Acta Biochim Pol. 2022 Nov 28;69(4):787-793. doi: 10.18388/abp.2020_6095. Acta Biochim Pol. 2022. PMID: 36441582
-
Cortical Neuron-Derived Exosomal MicroRNA-181c-3p Inhibits Neuroinflammation by Downregulating CXCL1 in Astrocytes of a Rat Model with Ischemic Brain Injury.Neuroimmunomodulation. 2019;26(5):217-233. doi: 10.1159/000502694. Epub 2019 Oct 30. Neuroimmunomodulation. 2019. Retraction in: Neuroimmunomodulation. 2023;30(1):184. doi: 10.1159/000531807. PMID: 31665717 Retracted.
-
Modulation of neuroplasticity and neuroinflammation by exosomal proteins and microRNA in depression: A review.Int J Biol Macromol. 2025 May;309(Pt 2):142829. doi: 10.1016/j.ijbiomac.2025.142829. Epub 2025 Apr 3. Int J Biol Macromol. 2025. PMID: 40187467 Review.
-
Glial and blood-brain barrier cell-derived exosomes: Implications in stroke.Microvasc Res. 2025 Jul;160:104812. doi: 10.1016/j.mvr.2025.104812. Epub 2025 Apr 15. Microvasc Res. 2025. PMID: 40246225 Review.
Cited by
-
Impact of Microglia-Derived Extracellular Vesicles on Resident Central Nervous System Cell Populations After Acute Brain Injury Under Various External Stimuli Conditions.Mol Neurobiol. 2025 Aug;62(8):9586-9603. doi: 10.1007/s12035-025-04858-w. Epub 2025 Mar 24. Mol Neurobiol. 2025. PMID: 40126599 Free PMC article. Review.
-
Neuroinflammation and energy metabolism: a dual perspective on ischemic stroke.J Transl Med. 2025 Apr 10;23(1):413. doi: 10.1186/s12967-025-06440-3. J Transl Med. 2025. PMID: 40211331 Free PMC article. Review.
-
Extracellular Vesicle-Derived miRNAs in Ischemic Stroke: Roles in Neuroprotection, Tissue Regeneration, and Biomarker Potential.Cell Mol Neurobiol. 2025 Mar 31;45(1):31. doi: 10.1007/s10571-025-01551-3. Cell Mol Neurobiol. 2025. PMID: 40164816 Free PMC article. Review.
-
Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go.Biomedicines. 2025 May 21;13(5):1262. doi: 10.3390/biomedicines13051262. Biomedicines. 2025. PMID: 40427089 Free PMC article. Review.
-
The Potential Role of Exosomes in Communication Between Astrocytes and Endothelial Cells.Int J Mol Sci. 2025 May 14;26(10):4676. doi: 10.3390/ijms26104676. Int J Mol Sci. 2025. PMID: 40429819 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

