Citrulline and ADI-PEG20 reduce inflammation in a juvenile porcine model of acute endotoxemia
- PMID: 39176089
- PMCID: PMC11338849
- DOI: 10.3389/fimmu.2024.1400574
Citrulline and ADI-PEG20 reduce inflammation in a juvenile porcine model of acute endotoxemia
Abstract
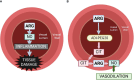
Background: Arginine is a conditionally essential amino acid that is depleted in critically ill or surgical patients. In pediatric and adult patients, sepsis results in an arginine-deficient state, and the depletion of plasma arginine is associated with greater mortality. However, direct supplementation of arginine can result in the excessive production of nitric oxide (NO), which can contribute to the hypotension and macrovascular hypo-reactivity observed in septic shock. Pegylated arginine deiminase (ADI-PEG20, pegargiminase) reduces plasma arginine and generates citrulline that can be transported intracellularly to generate local arginine and NO, without resulting in hypotension, while maintaining microvascular patency. The objective of this study was to assess the efficacy of ADI-PEG20 with and without supplemental intravenous citrulline in mitigating hypovolemic shock, maintaining tissue levels of arginine, and reducing systemic inflammation in an endotoxemic pediatric pig model.
Methods: Twenty 3-week-old crossbred piglets were implanted with jugular and carotid catheters as well as telemetry devices in the femoral artery to measure blood pressure, body temperature, heart rate, and respiration rate. The piglets were assigned to one of three treatments before undergoing a 5 h lipopolysaccharide (LPS) infusion protocol. Twenty-four hours before LPS infusion, control pigs (LPS; n=6) received saline, ADI-PEG20 pigs (n=7) received an injection of ADI-PEG20, and seven pigs (ADI-PEG20 + CIT pigs [n=7]) received ADI-PEG20 and 250 mg/kg citrulline intravenously. Pigs were monitored throughout LPS infusion and tissue was harvested at the end of the protocol.
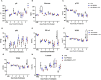
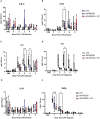
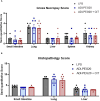
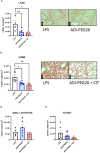
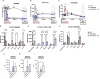
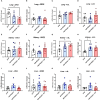
Results: Plasma arginine levels decreased and remained low in ADI-PEG20 + CIT and ADI-PEG20 pigs compared with LPS pigs but tissue arginine levels in the liver and kidney were similar across all treatments. Mean arterial pressure in all groups decreased from 90 mmHg to 60 mmHg within 1 h of LPS infusion but there were no significant differences between treatment groups. ADI-PEG20 and ADI-PEG20 + CIT pigs had less CD45+ infiltrate in the liver and lung and lower levels of pro-inflammatory cytokines in the plasma.
Conclusion: ADI-PEG20 and citrulline supplementation failed to ameliorate the hypotension associated with acute endotoxic sepsis in pigs but reduced systemic and local inflammation in the lung and liver.
Keywords: ADI-PEG20; NO; arginine; citrulline; sepsis.
Copyright © 2024 Vonderohe, Stoll, Didelija, Nguyen, Mohammad, Jones-Hall, Cruz, Marini and Burrin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

