Pulmonary neuroendocrine cells: crucial players in respiratory function and airway-nerve communication
- PMID: 39176384
- PMCID: PMC11340541
- DOI: 10.3389/fnins.2024.1438188
Pulmonary neuroendocrine cells: crucial players in respiratory function and airway-nerve communication
Abstract
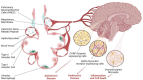
Pulmonary neuroendocrine cells (PNECs) are unique airway epithelial cells that blend neuronal and endocrine functions, acting as key sensors in the lung. They respond to environmental stimuli like allergens by releasing neuropeptides and neurotransmitters. PNECs stand out as the only lung epithelial cells innervated by neurons, suggesting a significant role in airway-nerve communication via direct neural pathways and hormone release. Pathological conditions such as asthma are linked to increased PNECs counts and elevated calcitonin gene-related peptide (CGRP) production, which may affect neuroprotection and brain function. CGRP is also associated with neurodegenerative diseases, including Parkinson's and Alzheimer's, potentially due to its influence on inflammation and cholinergic activity. Despite their low numbers, PNECs are crucial for a wide range of functions, highlighting the importance of further research. Advances in technology for producing and culturing human PNECs enable the exploration of new mechanisms and cell-specific responses to targeted therapies for PNEC-focused treatments.
Keywords: HPSC; IPSC; brain; lung; pulmonary neuroendocrine cells; stem cells.
Copyright © 2024 Thakur, Mei, Zhang, Zhang, Taslakjian, Lian, Wu, Chen, Solway and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- An Q., Sun C., Li R., Chen S., Gu X., An S., et al. (2021). Calcitonin gene-related peptide regulates spinal microglial activation through the histone H3 lysine 27 trimethylation via enhancer of zeste homolog-2 in rats with neuropathic pain. J. Neuroinflammation 18 117. 10.1186/s12974-021-02168-1 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

