Targeting TREX1 Induces Innate Immune Response in Drug-Resistant Small-Cell Lung Cancer
- PMID: 39177280
- PMCID: PMC11391691
- DOI: 10.1158/2767-9764.CRC-24-0360
Targeting TREX1 Induces Innate Immune Response in Drug-Resistant Small-Cell Lung Cancer
Abstract
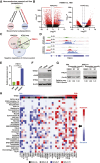
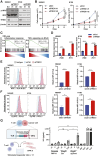
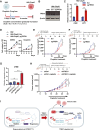
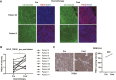
Small-cell lung cancer (SCLC) is the most lethal type of lung cancer. Paradoxically, this tumor displays an initial exquisite response to chemotherapy; however, at relapse, the tumor is highly resistant to subsequent available therapies. Here, we report that the expression of three prime repair exonuclease 1 (TREX1) is strongly induced in chemoresistant SCLCs. Assay for transposase-accessible chromatin using sequencing and chromatin immunoprecipitation sequencing revealed a significant increase in chromatin accessibility and transcriptional activity of TREX1 gene locus in chemoresistant SCLCs. Analyses of human SCLC tumors and patient-derived xenografts (PDX) also showed an increase in TREX1 expression in postchemotherapy samples. TREX1 depletion caused the activation of cyclic GMP-AMP synthase stimulator of interferon gene pathway due to cytoplasmic accumulation of damage-associated double-stranded DNA, inducing immunogenicity and enhancing the sensitivity of drug-resistant cells to chemotherapy. These findings suggest TREX1 upregulation may partially contribute to the survival of resistant cells, and its inhibition may represent a promising therapeutic strategy to enhance antitumor immunity and potentiate the efficacy of chemotherapy and/or immunotherapy in chemoresistant SCLCs. Significance: In this study, we show that targeting TREX1 induces an innate immune response and resensitizes SCLC cells to chemotherapy, representing a promising novel target for "immunologically" cold tumors, such as SCLC.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
N.R. Mahadevan reports ownership of stocks in AstraZeneca and Roche. E.H. Knelson reports personal fees from Merck & Co outside the submitted work. Y.P. Hung reports personal fees from Elsevier and the American Society of Clinical Pathology outside the submitted work. A.N. Hata reports grants and personal fees from Amgen; grants from BridgeBio, Bristol Myers Squibb, C4 Therapeutics, Eli Lilly, and Novartis; grants and personal fees from Nuvalent and Pfizer; grants from Scorpion Therapeutics; and personal fees from Engine Biosciences, Oncovalent, TigaTx, and Tolremo Therapeutics outside the submitted work. D.A. Barbie reports personal fees from Qiagen/N of One, other support from Xsphera Biosciences, grants from Gilead Sciences and Novartis, and personal fees from Nerviano Medical Sciences during the conduct of the study. No disclosures were reported by the other authors.
Figures

References
-
- Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. - PubMed
-
- Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

