Long-Lasting Response to Lorlatinib in Patients with ALK-Driven Relapsed or Refractory Neuroblastoma Monitored with Circulating Tumor DNA Analysis
- PMID: 39177282
- PMCID: PMC11440348
- DOI: 10.1158/2767-9764.CRC-24-0338
Long-Lasting Response to Lorlatinib in Patients with ALK-Driven Relapsed or Refractory Neuroblastoma Monitored with Circulating Tumor DNA Analysis
Abstract
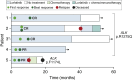
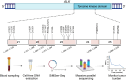
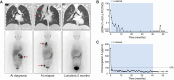
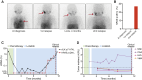
Patients with anaplastic lymphoma kinase (ALK)-driven neuroblastoma may respond to tyrosine kinase inhibitors, but resistance to treatment occurs and methods currently used for detection of residual disease have limited sensitivity. Here, we present a national unselected cohort of five patients with relapsed or refractory ALK-driven neuroblastoma treated with lorlatinib as monotherapy and test the potential of targeted circulating tumor DNA (ctDNA) analysis as a guide for treatment decisions in these patients. We developed a sequencing panel for ultrasensitive detection of ALK mutations associated with neuroblastoma or resistance to tyrosine kinase inhibitors and used it for ctDNA analysis in 83 plasma samples collected longitudinally from the four patients who harbored somatic ALK mutations. All four patients with ALK p.R1275Q experienced major responses and were alive 35 to 61 months after starting lorlatinib. A fifth patient with ALK p.F1174L initially had a partial response but relapsed after 10 months of treatment. In all cases, ctDNA was detected at the start of lorlatinib single-agent treatment and declined gradually, correlating with clinical responses. In the two patients exhibiting relapse, ctDNA increased 9 and 3 months, respectively, before clinical detection of disease progression. In one patient harboring HRAS p.Q61L in the relapsed tumor, retrospective ctDNA analysis showed that the mutation appeared de novo after 8 months of lorlatinib treatment. We conclude that some patients with relapsed or refractory high-risk neuroblastoma show durable responses to lorlatinib as monotherapy, and targeted ctDNA analysis is effective for evaluation of treatment and early detection of relapse in ALK-driven neuroblastoma.
Significance: We present five patients with ALK-driven relapsed or refractory neuroblastoma treated with lorlatinib as monotherapy. All patients responded to treatment, and four of them were alive after 3 to 5 years of follow-up. We performed longitudinal ctDNA analysis with ultra-deep sequencing of the ALK tyrosine kinase domain. We conclude that ctDNA analysis may guide treatment decisions in ALK-driven neuroblastoma, also when the disease is undetectable using standard clinical methods.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Ståhlberg reports grants from the Swedish Cancer Society, the Swedish Childhood Cancer Foundation, the Swedish Research Council, the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement, Sweden’s Innovation Agency, the Sjöberg Foundation, Region Västra Götaland, and the Sarcoma patient organization research fund, the memorial fund of Elvira Trané; grants and personal fees from the University of Gothenburg; and personal fees from the Sahlgrenska University Hospital during the conduct of the study; as well as other support from Iscaffpharma, Simsen Diagnostics, and Tulebovaasta outside the submitted work; and a patent to simple, multiplexed, PCR-based barcoding of DNA for sensitive mutation detection using sequencing issued. P. Kogner reports personal fees from Recordati outside the submitted work. M. Dalin reports grants from the Wallenberg Centre for Molecular and Translational Medicine, the Swedish Childhood Cancer Fund, the Swedish Society for Medical Research, the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement, Sahlgrenska Academy at the University of Gothenburg, the Assar Gabrielsson Research Foundation, and Svensson’s Fund for Medical Research during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
-
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 2014;64:83–103. - PubMed
-
- Mosse YP, Diskin SJ, Wasserman N, Rinaldi K, Attiyeh EF, Cole K, et al. . Neuroblastomas have distinct genomic DNA profiles that predict clinical phenotype and regional gene expression. Genes Chromosomes Cancer 2007;46:936–49. - PubMed
-
- Ladenstein R, Pötschger U, Pearson ADJ, Brock P, Luksch R, Castel V, et al. . Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 2017;18:500–14. - PubMed
-
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. . Neuroblastoma. Nat Rev Dis Primers 2016;2:16078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

