A behind-the-scenes role of BDNF in the survival and differentiation of spermatogonia
- PMID: 39177410
- PMCID: PMC11784946
- DOI: 10.4103/aja202457
A behind-the-scenes role of BDNF in the survival and differentiation of spermatogonia
Abstract
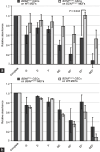
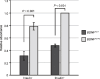
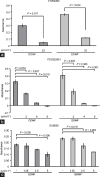
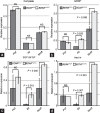
Mouse spermatogenesis entails the maintenance and self-renewal of spermatogonial stem cells (SSCs), which require a complex web-like signaling network transduced by various cytokines. Although brain-derived neurotrophic factor (BDNF) is expressed in Sertoli cells in the testis, and its receptor tropomyosin receptor kinase B (TrkB) is expressed in the spermatogonial population containing SSCs, potential functions of BDNF for spermatogenesis have not been uncovered. Here, we generate BDNF conditional knockout mice and find that BDNF is dispensable for in vivo spermatogenesis and fertility. However, in vitro , we reveal that BDNF -deficient germline stem cells (GSCs) exhibit growth potential not only in the absence of glial cell line-derived neurotrophic factor (GDNF), a master regulator for GSC proliferation, but also in the absence of other factors, including epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and insulin. GSCs grown without these factors are prone to differentiation, yet they maintain expression of promyelocytic leukemia zinc finger ( Plzf ), an undifferentiated spermatogonial marker. Inhibition of phosphoinositide 3-kinase (PI3K), mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), and Src pathways all interfere with the growth of BDNF-deficient GSCs. Thus, our findings suggest a role for BDNF in maintaining the undifferentiated state of spermatogonia, particularly in situations where there is a shortage of growth factors.
Copyright ©The Author(s)(2024).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
-
- Kanatsu-Shinohara M, Shinohara T. Spermatogonial stem cell self-renewal and development. Annu Rev Cell Dev Biol. 2013;29:163–87. - PubMed
-
- Russell LD. Histological and Histopathological Evaluation of the Testis. Clearwater: Cache River Press; 1990.
-
- O’Donnell L, O’Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. - PubMed
-
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–6. - PubMed
-
- Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, et al. Improved serum- and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014;91:88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

