Formation of Nanodiamonds during Pyrolysis of Butanosolv Lignin
- PMID: 39177501
- PMCID: PMC11394345
- DOI: 10.1021/acsnano.4c02950
Formation of Nanodiamonds during Pyrolysis of Butanosolv Lignin
Abstract
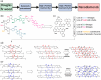
The preparation of artificial diamonds has a long history driven by decreased costs compared to naturally occurring diamonds and ethical issues. However, fabrication of diamonds in the laboratory from readily available biomass has not been extensively investigated. This work demonstrates a convenient method for producing nanodiamonds from biopolymer lignin at ambient pressure. Lignin was extracted from Douglas Fir sawdust using a butanosolv pretreatment and was pyrolyzed in N2 at 1000 °C, followed by a second thermal treatment in 5% H2/Ar at 1050 °C, both at ambient pressure. This led to the formation of nanodiamonds embedded in an amorphous carbon substrate. The changes occurring at various stages of the pyrolysis process were monitored by scanning electron microscopy, Fourier transform infrared spectroscopy, and nuclear magnetic resonance spectroscopy. High resolution transmission electron microscopy revealed that nanodiamond crystallites, 4 nm in diameter on average, formed via multiple nucleation events in some carbon-containing high density spheres. It is proposed that highly defected graphene-like flakes form during the pyrolysis of lignin as an intermediate phase. These flakes are more deformable with more localized π electrons in comparison with graphene and join together face-to-face in different manners to form cubic or hexagonal nanodiamonds. This proposed mechanism for the formation of nanodiamonds is relevant to the future fabrication of diamonds from biomass under relatively mild conditions.
Keywords: electron microscopy; lignin; multiple nucleation; nanodiamond; pyrolysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Liu W.-J.; Jiang H.; Yu H.-Q. Thermochemical Conversion of Lignin to Functional Materials: a Review and Future Directions. Green Chem. 2015, 17, 4888–4907. 10.1039/C5GC01054C. - DOI
-
- Abbaschian R.; Zhu H.; Clarke C. High Pressure-High Temperature Growth of Diamond Crystals Using Split Sphere Apparatus. Diamond Relat. Mater. 2005, 14, 1916–1919. 10.1016/j.diamond.2005.09.007. - DOI
-
- Iijima S.; Ichihashi T. Single-Shell Carbon Nanotubes of 1-nm Diameter. Nature 1993, 363, 603–605. 10.1038/363603a0. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

