Patient selection, ventricular tachycardia substrate delineation, and data transfer for stereotactic arrhythmia radioablation: a clinical consensus statement of the European Heart Rhythm Association of the European Society of Cardiology and the Heart Rhythm Society
- PMID: 39177652
- PMCID: PMC12041921
- DOI: 10.1093/europace/euae214
Patient selection, ventricular tachycardia substrate delineation, and data transfer for stereotactic arrhythmia radioablation: a clinical consensus statement of the European Heart Rhythm Association of the European Society of Cardiology and the Heart Rhythm Society
Abstract
Stereotactic arrhythmia radioablation (STAR) is a novel, non-invasive, and promising treatment option for ventricular arrhythmias (VAs). It has been applied in highly selected patients mainly as bailout procedure, when (multiple) catheter ablations, together with anti-arrhythmic drugs, were unable to control the VAs. Despite the increasing clinical use, there is still limited knowledge of the acute and long-term response of normal and diseased myocardium to STAR. Acute toxicity appeared to be reasonably low, but potential late adverse effects may be underreported. Among published studies, the provided methodological information is often limited, and patient selection, target volume definition, methods for determination and transfer of target volume, and techniques for treatment planning and execution differ across studies, hampering the pooling of data and comparison across studies. In addition, STAR requires close and new collaboration between clinical electrophysiologists and radiation oncologists, which is facilitated by shared knowledge in each collaborator's area of expertise and a common language. This clinical consensus statement provides uniform definition of cardiac target volumes. It aims to provide advice in patient selection for STAR including aetiology-specific aspects and advice in optimal cardiac target volume identification based on available evidence. Safety concerns and the advice for acute and long-term monitoring including the importance of standardized reporting and follow-up are covered by this document. Areas of uncertainty are listed, which require high-quality, reliable pre-clinical and clinical evidence before the expansion of STAR beyond clinical scenarios in which proven therapies are ineffective or unavailable.
Keywords: Ablation; Radiotherapy; Stereotactic arrhythmia radioablation (STAR); Sudden death; Ventricular tachycardia.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures

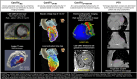
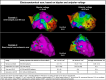
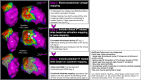
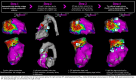

References
-
- Grehn M, Mandija S, Miszczyk M, Krug D, Tomasik B, Stickney KE et al. STereotactic Arrhythmia Radioablation (STAR): the Standardized Treatment and Outcome Platform for Stereotactic Therapy Of Re-entrant tachycardia by a Multidisciplinary consortium (STOPSTORM.eu) and review of current patterns of STAR practice in Europe. Europace 2023;25:1284–95. - PMC - PubMed
-
- Chang SD, Main W, Martin DP, Gibbs IC, Heilbrun MP. An analysis of the accuracy of the CyberKnife: a robotic frameless stereotactic radiosurgical system. Neurosurgery 2003;52:140–6; discussion 6–7. - PubMed
-
- Kautzner J, Jedlickova K, Sramko M, Peichl P, Cvek J, Ing LK et al. Radiation-induced changes in ventricular myocardium after stereotactic body radiotherapy for recurrent ventricular tachycardia. JACC Clin Electrophysiol 2021;7:1487–92. - PubMed
-
- Neuwirth R, Cvek J, Knybel L, Jiravsky O, Molenda L, Kodaj M et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace 2019;21:1088–95. - PubMed

