Next-generation sequencing-based evaluation of the actionable landscape of genomic alterations in solid tumors: the "MOZART" prospective observational study
- PMID: 39177668
- PMCID: PMC11783315
- DOI: 10.1093/oncolo/oyae206
Next-generation sequencing-based evaluation of the actionable landscape of genomic alterations in solid tumors: the "MOZART" prospective observational study
Abstract
Background: The identification of the most appropriate targeted therapies for advanced cancers is challenging. We performed a molecular profiling of metastatic solid tumors utilizing a comprehensive next-generation sequencing (NGS) assay to determine genomic alterations' type, frequency, actionability, and potential correlations with PD-L1 expression.
Methods: A total of 304 adult patients with heavily pretreated metastatic cancers treated between January 2019 and March 2021 were recruited. The CLIA-/UKAS-accredit Oncofocus assay targeting 505 genes was used on newly obtained or archived biopsies. Chi-square, Kruskal-Wallis, and Wilcoxon rank-sum tests were used where appropriate. Results were significant for P < .05.
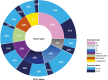
Results: A total of 237 tumors (78%) harbored potentially actionable genomic alterations. Tumors were positive for PD-L1 in 68.9% of cases. The median number of mutant genes/tumor was 2.0 (IQR: 1.0-3.0). Only 34.5% were actionable ESCAT Tier I-II with different prevalence according to cancer type. The DNA damage repair (14%), the PI3K/AKT/mTOR (14%), and the RAS/RAF/MAPK (12%) pathways were the most frequently altered. No association was found among PD-L1, ESCAT, age, sex, and tumor mutational status. Overall, 62 patients underwent targeted treatment, with 37.1% obtaining objective responses. The same molecular-driven treatment for different cancer types could be associated with opposite clinical outcomes.
Conclusions: We highlight the clinical value of molecular profiling in metastatic solid tumors using comprehensive NGS-based panels to improve treatment algorithms in situations of uncertainty and facilitate clinical trial recruitment. However, interpreting genomic alterations in a tumor type-specific manner is critical.
Keywords: ESCAT; clinical actionability; metastatic; molecular profiling; next-generation sequencing; solid tumors.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
Francesco Schettini reports honoraria from Novartis, Gilead, and Daiichy-Sankyo for educational events/materials and travel expenses from Novartis, Gilead, and Daiichy-Sankyo. Daniele Generali declares personal fees for educational events by Novartis, Lilly, Pfizer, Daiichy-Sankyo, Roche; research funds from Astrazeneca, Novartis, and LILT. Marco Loddo, Gareth H Williams, Keeda-Marie Hardisty, Paul Scorer, and Robert Thatcher are employees of Oncologica UK Ltd. Maurizio Scaltriti is an employee of AstraZeneca. The other authors have nothing to declare.
Figures


References
-
- Allemani C, Matsuda T, Di Carlo V, et al.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. 10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed
-
- Loddo M, Hardisty K-M, Thatcher RP, Haddow TE, Williams GH.. The actionable genomic mutational landscape in solid tumours. JCO. 2020;38(15_suppl):e13642-e13642. 10.1200/jco.2020.38.15_suppl.e13642 - DOI
-
- National Comprehensive Cancer Network. NCCN Guidelines for Melanoma: Cutaneous, version 2; 2024. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed April 4, 2024. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

