Immune Cell Contribution to Mammary Gland Development
- PMID: 39177859
- PMCID: PMC11343902
- DOI: 10.1007/s10911-024-09568-y
Immune Cell Contribution to Mammary Gland Development
Abstract
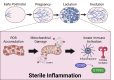
Postpartum breast cancer (PPBC) is a unique subset of breast cancer, accounting for nearly half of the women diagnosed during their postpartum years. Mammary gland involution is widely regarded as being a key orchestrator in the initiation and progression of PPBC due to its unique wound-healing inflammatory signature. Here, we provide dialogue suggestive that lactation may also facilitate neoplastic development as a result of sterile inflammation. Immune cells are involved in all stages of postnatal mammary development. It has been proposed that the functions of these immune cells are partially directed by mammary epithelial cells (MECs) and the cytokines they produce. This suggests that a more niche area of exploration aimed at assessing activation of innate immune pathways within MECs could provide insight into immune cell contributions to the developing mammary gland. Immune cell contribution to pubertal development and mammary gland involution has been extensively studied; however, investigations into pregnancy and lactation remain limited. During pregnancy, the mammary gland undergoes dramatic expansion to prepare for lactation. As a result, MECs are susceptible to replicative stress. During lactation, mitochondria are pushed to capacity to fulfill the high energetic demands of producing milk. This replicative and metabolic stress, if unresolved, can elicit activation of innate immune pathways within differentiating MECs. In this review, we broadly discuss postnatal mammary development and current knowledge of immune cell contribution to each developmental stage, while also emphasizing a more unique area of study that will be beneficial in the discovery of novel therapeutic biomarkers of PPBC.
Keywords: Adaptive immunity; Inflammation; Innate immunity; Lactation; Mammary gland development; Postpartum breast cancer; Pregnancy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, Urquhart A, Schedin P, Borges VF. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. 2013;138(2):549–59. 10.1007/s10549-013-2437-x. 10.1007/s10549-013-2437-x - DOI - PMC - PubMed
-
- Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, Michels KB, Palmer JR, Schedin P, Stuebe AM, Watson CJ, Sherman ME. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute-sponsored workshop. J Natl Cancer Inst. 2013;105(3):166–74. 10.1093/jnci/djs505. 10.1093/jnci/djs505 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

