Targeting Non-V600 Mutations in BRAF: A Single Institution Retrospective Analysis and Review of the Literature
- PMID: 39177935
- PMCID: PMC11455815
- DOI: 10.1007/s40268-024-00475-5
Targeting Non-V600 Mutations in BRAF: A Single Institution Retrospective Analysis and Review of the Literature
Abstract
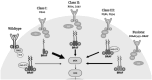
Background and objective: While successful treatment paradigms for BRAF V600 mutations have been developed, 10% of BRAF mutations are not at V600 and lack a standard treatment regimen. This study summarizes the current body of knowledge on the treatment of non-V600 mutations and reports a single institution experience.
Methods: We conducted a literature review to summarize relevant preclinical and clinical published data on the response of non-V600 mutations to targeted therapies. We performed a retrospective analysis of INOVA Schar Cancer patients registered in our Molecular Tumor Board database with non-V600 BRAF mutations who were recipients of targeted therapy and assessed their time to next treatment and best response.
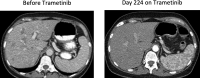
Results: Published preclinical and clinical data have demonstrated limiting results in the response of non-V600 mutated cancers to targeted therapies. Response rates were variable for the major classes of BRAF mutations including class II and class III mutations as well as, BRAF fusions. Data collected from our INOVA cohort offered promising results with one patient achieving partial remission and two patients achieving stable disease.
Conclusions: This article reflects the current understanding of targeted therapies in non-V600 mutations. Further large-scale studies separating BRAF mutations based on their mechanism of activation will expand our understanding.
© 2024. The Author(s).
Conflict of interest statement
Hirra A. Chaudhary declares no potential competing interests. Timothy Cannon is a paid Molecular Tumor Board member for Intermountain Health. He has not had any other consulting in 2022. In 2021, he consulted for Bayer and Deciphera (both less than $5000). Arthur Winer has ownership stock in Sciencella Honoraria, HalioDx, and OncLive.
Figures
References
-
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002. 10.1038/nature00766. - PubMed
-
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019. 10.1056/NEJMoa1904059. - PubMed
-
- US FDA. FDA grants regular approval to dabrafenib and trametinib combination for metastatic NSCLC with BRAF V600e mutation. Case medical research. 2017. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grant.... [Accessed 3 Jul 2024].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

