Tourniquet Use and Local Tissue Concentrations of Cefazolin During Total Knee Arthroplasty: A Randomized Clinical Trial
- PMID: 39178000
- PMCID: PMC11344230
- DOI: 10.1001/jamanetworkopen.2024.29702
Tourniquet Use and Local Tissue Concentrations of Cefazolin During Total Knee Arthroplasty: A Randomized Clinical Trial
Abstract
Importance: Prophylactic administration of antibiotics before skin incision is an important component in the prevention of periprosthetic joint infection in arthroplasty surgery. For antibiotics to be effective, the local tissue concentration (LTC) must exceed the minimum inhibitory concentration of typical infecting organisms; however, the LTC of cefazolin during arthroplasty is poorly understood.
Objective: To compare the systemic concentration of cefazolin in serum with the LTC in fat, synovium, and bone during primary total knee arthroplasty (TKA) while assessing the effect of tourniquet inflation.
Design, setting, and participants: This prospective randomized clinical trial was conducted from March 1, 2022, to June 30, 2023, in patients undergoing TKA at a single academic center.
Intervention: Total knee arthroplasty with or without a limb tourniquet.
Main outcomes and measures: Systemic blood and local tissues from the surgical site (fat, synovium, and bone) were harvested at regular intervals during the surgery. The primary outcome was the LTC of cefazolin, quantified using the liquid chromatography-tandem mass spectrometry technique.
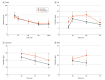
Results: A total of 59 patients were included in the study, with 29 in the tourniquet group (mean [SD] age, 69.3 [9.6] years; 23 [79.3%] female) and 30 in the no tourniquet group (mean [SD] age, 69.9 [9.7] years; 21 [70.0%] female). In patients undergoing TKA without a tourniquet, the mean concentration of cefazolin in serum was 71.9 μg/mL (95% CI, 66.4-77.5 μg/mL), whereas the mean LTCs were 13.9 μg/g (95% CI, 12.1-15.7 μg/g) in fat, 27.7 μg/g (95% CI, 24.3-31.0 μg/g) in synovium, and 17.7 μg/g (95% CI, 14.8-20.5 μg/g) in bone. For patients undergoing TKA with a tourniquet, the mean concentration of cefazolin in serum was 72.0 μg/mL (95% CI, 66.3-77.7 μg/mL), and the mean LTCs were 9.9 μg/g (95% CI, 8.7-11.1 μg/g) in fat, 21.8 μg/g (95% CI, 18.7-25.0 μg/g) in synovium, and 13.0 μg/g (95% CI, 10.8-15.2 μg/g) in bone. The use of a tourniquet resulted in significantly lower mean LTCs by 60 minutes after cefazolin infusion (10.8 μg/g [95% CI, 9.1-12.4 μg/g] vs 16.9 μg/g [95% CI, 14.1-19.6 μg/g], P = .001 in fat; 18.9 μg/g [95% CI, 14.1-23.6 μg/g] vs 25.8 μg/g [95% CI, 21.4-30.3 μg/g], P = .03 in synovium; and 11.8 μg/g [95% CI, 9.3-14.2 μg/g] vs 19.4 μg/g [95% CI, 14.5-24.4 μg/g], P = .007 in bone).
Conclusions and relevance: In this randomized clinical trial, the concentration of cefazolin was lower in local tissues (fat, synovium, and bone) than in systemic blood, and the use of a limb tourniquet further significantly reduced these concentrations. Although the current prophylactic dosing regimen for cefazolin provides sufficient serum concentrations, the levels in the periarticular tissue during TKA may be insufficient to prevent periprosthetic joint infection.
Trial registration: ClinicalTrials.gov Identifier: NCT05604157.
Conflict of interest statement
Figures
References
-
- Canadian Institute for Health Information . Hip and knee replacements in Canada, 2017-2018: Canadian Joint Replacement Registry annual report. Accessed July 8, 2024. https://secure.cihi.ca/free_products/cjrr-annual-report-2019-en-web.pdf
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical