Cells and circuits for amygdala neuroplasticity in the transition to chronic pain
- PMID: 39178115
- PMCID: PMC11473139
- DOI: 10.1016/j.celrep.2024.114669
Cells and circuits for amygdala neuroplasticity in the transition to chronic pain
Abstract
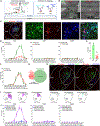
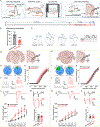
Maladaptive plasticity is linked to the chronification of diseases such as pain, but the transition from acute to chronic pain is not well understood mechanistically. Neuroplasticity in the central nucleus of the amygdala (CeA) has emerged as a mechanism for sensory and emotional-affective aspects of injury-induced pain, although evidence comes from studies conducted almost exclusively in acute pain conditions and agnostic to cell type specificity. Here, we report time-dependent changes in genetically distinct and projection-specific CeA neurons in neuropathic pain. Hyperexcitability of CRF projection neurons and synaptic plasticity of parabrachial (PB) input at the acute stage shifted to hyperexcitability without synaptic plasticity in non-CRF neurons at the chronic phase. Accordingly, chemogenetic inhibition of the PB→CeA pathway mitigated pain-related behaviors in acute, but not chronic, neuropathic pain. Cell-type-specific temporal changes in neuroplasticity provide neurobiological evidence for the clinical observation that chronic pain is not simply the prolonged persistence of acute pain.
Keywords: CP: Neuroscience; CRF; amygdala; corticotropin-releasing factor; electrophysiology; neuropathic pain; neuroplasticity; pain mechanisms; parabrachial nucleus; protein kinase C delta; somatostatin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Breivik H, Eisenberg E, and O’Brien T; OPENMinds (2013). The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Publ. Health 13, 1229. 10.1186/1471-2458-13-1229. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

