Phosphatidylinositol-3-phosphate mediates Arc capsid secretion through the multivesicular body pathway
- PMID: 39178227
- PMCID: PMC11363301
- DOI: 10.1073/pnas.2322422121
Phosphatidylinositol-3-phosphate mediates Arc capsid secretion through the multivesicular body pathway
Erratum in
-
Correction for Mehta et al., Phosphatidylinositol-3-phosphate mediates Arc capsid secretion through the multivesicular body pathway.Proc Natl Acad Sci U S A. 2024 Nov 12;121(46):e2420988121. doi: 10.1073/pnas.2420988121. Epub 2024 Nov 4. Proc Natl Acad Sci U S A. 2024. PMID: 39495933 Free PMC article. No abstract available.
Abstract
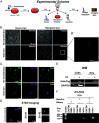
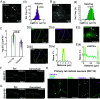
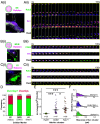
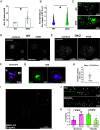
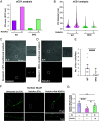
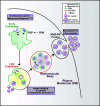
Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) is an immediate early gene that plays a vital role in learning and memory. Arc protein has structural and functional properties similar to viral Group-specific antigen (Gag) protein and mediates the intercellular RNA transfer through virus-like capsids. However, the regulators and secretion pathway through which Arc capsids maneuver cargos are unclear. Here, we identified that phosphatidylinositol-3-phosphate (PI3P) mediates Arc capsid assembly and secretion through the endosomal-multivesicular body (MVB) pathway. Indeed, reconstituted Arc protein preferably binds to PI3P. In HEK293T cells, Arc forms puncta that colocalize with FYVE, an endosomal PI3P marker, as well as Rab5 and CD63, early endosomal and MVB markers, respectively. Superresolution imaging resolves Arc accumulates within the intraluminal vesicles of MVB. CRISPR double knockout of RalA and RalB, crucial GTPases for MVB biogenesis and exocytosis, severely reduces the Arc-mediated RNA transfer efficiency. RalA/B double knockdown in cultured rat cortical neurons increases the percentage of mature dendritic spines. Intake of extracellular vesicles purified from Arc-expressing wild-type, but not RalA/B double knockdown, cells in mouse cortical neurons reduces their surface GlutA1 levels. These results suggest that unlike the HIV Gag, whose membrane targeting requires interaction with plasma-membrane-specific phosphatidyl inositol (4,5) bisphosphate (PI(4,5)P2), the assembly of Arc capsids is mediated by PI3P at endocytic membranes. Understanding Arc's secretion pathway helps gain insights into its role in intercellular cargo transfer and highlights the commonality and distinction of trafficking mechanisms between structurally resembled capsid proteins.
Keywords: activity-regulated cytoskeleton-associated protein; intercellular RNA transfer; multivesicular body; phospholipids; virus-like capsid.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Update of
-
Phosphatidylinositol 3-phosphate mediates Arc capsids secretion through the multivesicular body pathway.bioRxiv [Preprint]. 2023 Dec 20:2023.12.19.572392. doi: 10.1101/2023.12.19.572392. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2322422121. doi: 10.1073/pnas.2322422121. PMID: 38187623 Free PMC article. Updated. Preprint.
References
-
- Lynch C., Tristem M., A co-opted gypsy-type LTR-retrotransposon is conserved in the genomes of humans, sheep, mice, and rats. Curr. Biol. 13, 1518–1523 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

