L-type Ca2+ channel activation of STIM1-Orai1 signaling remodels the dendritic spine ER to maintain long-term structural plasticity
- PMID: 39178228
- PMCID: PMC11363309
- DOI: 10.1073/pnas.2407324121
L-type Ca2+ channel activation of STIM1-Orai1 signaling remodels the dendritic spine ER to maintain long-term structural plasticity
Abstract
Learning and memory require coordinated structural and functional plasticity at neuronal glutamatergic synapses located on dendritic spines. Here, we investigated how the endoplasmic reticulum (ER) controls postsynaptic Ca2+ signaling and long-term potentiation of dendritic spine size, i.e., sLTP that accompanies functional strengthening of glutamatergic synaptic transmission. In most ER-containing (ER+) spines, high-frequency optical glutamate uncaging (HFGU) induced long-lasting sLTP that was accompanied by a persistent increase in spine ER content downstream of a signaling cascade engaged by N-methyl-D-aspartate receptors (NMDARs), L-type Ca2+ channels (LTCCs), and Orai1 channels, the latter being activated by stromal interaction molecule 1 (STIM1) in response to ER Ca2+ release. In contrast, HFGU stimulation of ER-lacking (ER-) spines expressed only transient sLTP and exhibited weaker Ca2+ signals noticeably lacking Orai1 and ER contributions. Consistent with spine ER regulating structural metaplasticity, delivery of a second stimulus to ER- spines induced ER recruitment along with persistent sLTP, whereas ER+ spines showed no additional increases in size or ER content in response to sequential stimulation. Surprisingly, the physical interaction between STIM1 and Orai1 induced by ER Ca2+ release, but not the resulting Ca2+ entry through Orai1 channels, proved necessary for the persistent increases in both spine size and ER content required for expression of long-lasting late sLTP.
Keywords: long-term potentiation; store-operated Ca2+ channels; stromal interaction molecule 1; voltage-gated Ca2+ channels.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
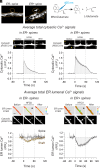

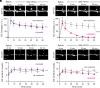
 ) versus MK801 (
) versus MK801 ( ), nimodipine (
), nimodipine ( ), and ryanodine (
), and ryanodine ( ) treated ER+ spines. All treatments significantly block ER Ca2+ depletion. (D) Mechanism for HFGU-evoked ER Ca2+ signals in ER+ spines.
) treated ER+ spines. All treatments significantly block ER Ca2+ depletion. (D) Mechanism for HFGU-evoked ER Ca2+ signals in ER+ spines.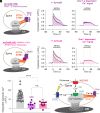
 ) and Orai1-dependent Ca2+ signals (
) and Orai1-dependent Ca2+ signals ( ) compared to total cytosolic Ca2+ signals (
) compared to total cytosolic Ca2+ signals ( ). Both Orai1,2-dependent and Orai1-dependent Ca2+ signals are significantly smaller than untreated total cytosolic Ca2+ signals, but Orai1,2-dependent and Orai1-dependent Ca2+ signals are not significantly different. (H) Graphical illustration of signal enhancement in ER+ spines.
). Both Orai1,2-dependent and Orai1-dependent Ca2+ signals are significantly smaller than untreated total cytosolic Ca2+ signals, but Orai1,2-dependent and Orai1-dependent Ca2+ signals are not significantly different. (H) Graphical illustration of signal enhancement in ER+ spines.
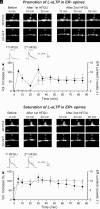
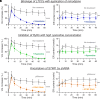
References
-
- Sala C., Segal M., Dendritic spines: The locus of structural and functional plasticity. Physiol. Rev. 94, 141–188 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

