Platelet-activating factor (PAF) promotes immunosuppressive neutrophil differentiation within tumors
- PMID: 39178229
- PMCID: PMC11363292
- DOI: 10.1073/pnas.2406748121
Platelet-activating factor (PAF) promotes immunosuppressive neutrophil differentiation within tumors
Abstract
Chronic inflammatory milieu in the tumor microenvironment (TME) leads to the recruitment and differentiation of myeloid-derived suppressor cells (MDSCs). Polymorphonuclear (PMN)-MDSCs, which are phenotypically and morphologically defined as a subset of neutrophils, cause major immune suppression in the TME, posing a significant challenge in the development of effective immunotherapies. Despite recent advances in our understanding of PMN-MDSC functions, the mechanism that gives rise to immunosuppressive neutrophils within the TME remains elusive. Both in vivo and in vitro, newly recruited neutrophils into the tumor sites remained activated and highly motile for several days and developed immunosuppressive phenotypes, as indicated by increased arginase 1 (Arg1) and dcTrail-R1 expression and suppressed anticancer CD8 T cell cytotoxicity. The strong suppressive function was successfully recapitulated by incubating naive neutrophils with cancer cell culture supernatant in vitro. Cancer metabolite secretome analyses of the culture supernatant revealed that both murine and human cancers released lipid mediators to induce the differentiation of immunosuppressive neutrophils. Liquid chromatography-mass spectrometry (LC-MS) lipidomic analysis identified platelet-activation factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) as a common tumor-derived lipid mediator that induces neutrophil differentiation. Lysophosphatidylcholine acyltransferase 2 (LPCAT2), the PAF biosynthetic enzyme, is up-regulated in human pancreatic ductal adenocarcinoma (PDAC) and shows an unfavorable correlation with patient survival across multiple cancer types. Our study identifies PAF as a lipid-driven mechanism of MDSC differentiation in the TME, providing a potential target for cancer immunotherapy.
Keywords: Cancer; MDSC; myeloid cells; neutrophil; tumor microenvironment.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

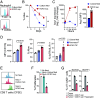


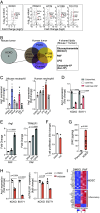

References
-
- Gabitass R. F., Annels N. E., Stocken D. D., Pandha H. A., Middleton G. W., Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother 60, 1419–1430 (2011). - PMC - PubMed
MeSH terms
Substances
Grants and funding
- Al147362/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- Al102851/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- GM007356/HHS | NIH | National Institute of General Medical Sciences (NIGMS)
- HL160723/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- R01 HL160723/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

