Structure-Activity Relationship Studies of the Peptide Antibiotic Clovibactin
- PMID: 39178334
- PMCID: PMC11382152
- DOI: 10.1021/acs.joc.4c01414
Structure-Activity Relationship Studies of the Peptide Antibiotic Clovibactin
Erratum in
-
Correction to "Structure-Activity Relationship Studies of the Peptide Antibiotic Clovibactin".J Org Chem. 2024 Oct 4;89(19):14608. doi: 10.1021/acs.joc.4c02243. Epub 2024 Sep 25. J Org Chem. 2024. PMID: 39320272 Free PMC article. No abstract available.
Abstract
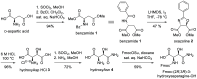
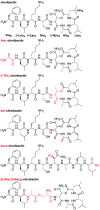
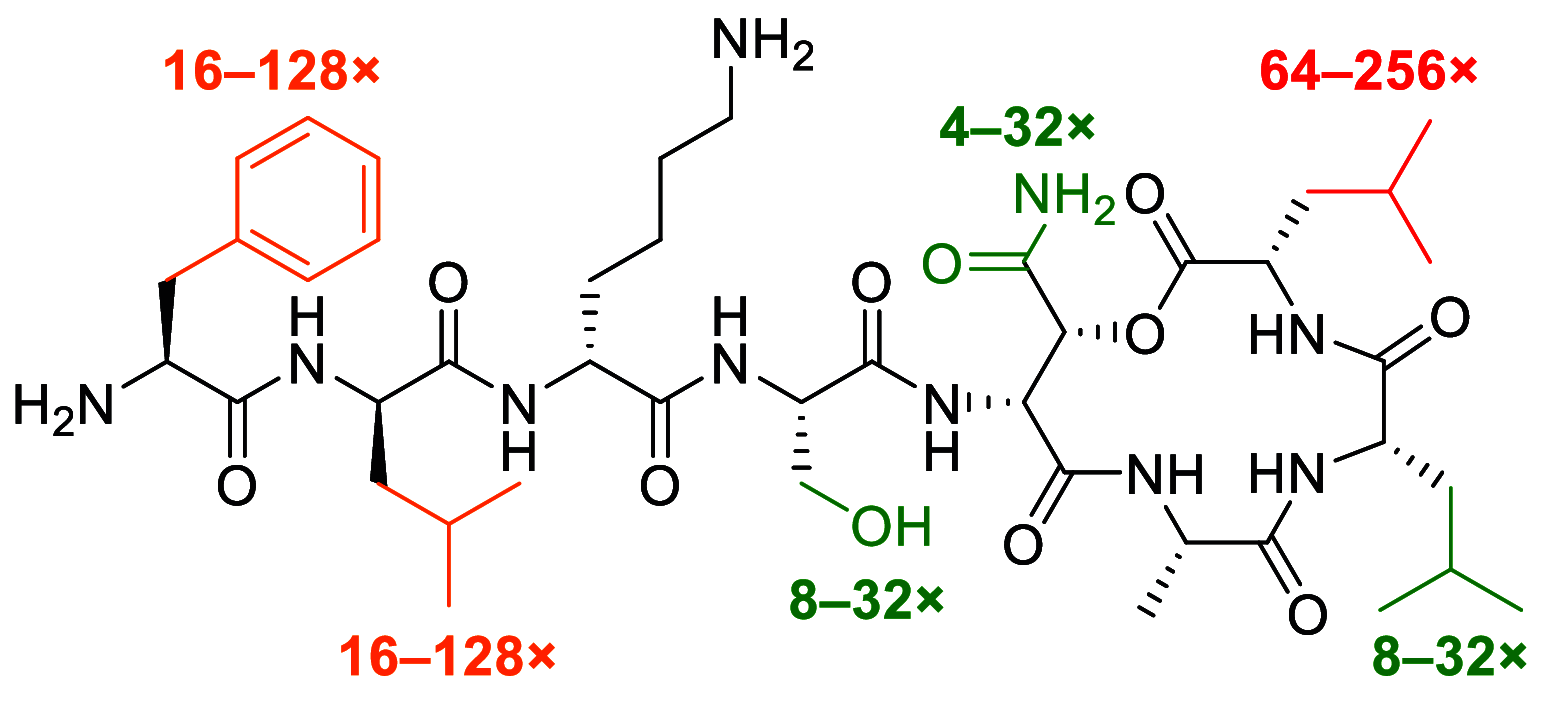
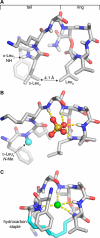
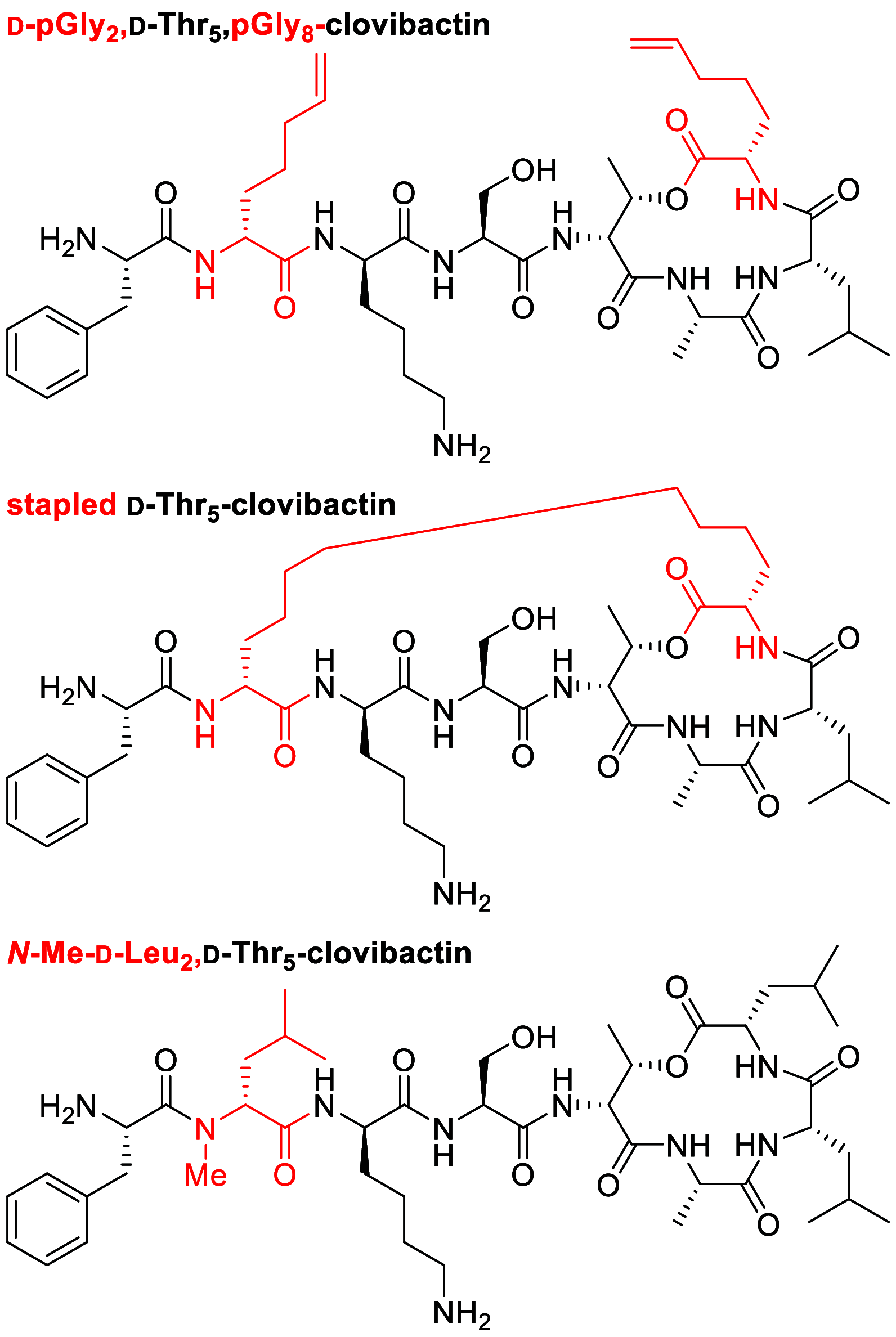
Our laboratory reported the chemical synthesis and stereochemical assignment of the recently discovered peptide antibiotic clovibactin. The current paper reports an improved, gram-scale synthesis of the amino acid building block Fmoc-(2R,3R)-3-hydroxyasparagine-OH that enables structure-activity relationship studies of clovibactin. An alanine scan reveals that residues Phe1, d-Leu2, Ser4, Leu7, and Leu8 are important for antibiotic activity. The side-chain amide group of the rare d-Hyn5 residue is not essential to activity and can be replaced with a methyl group with a moderate loss of activity. An acyclic clovibactin analogue reveals that the macrolactone ring is essential to antibiotic activity. The enantiomer of clovibactin is active, albeit somewhat less so than clovibactin. A conformationally constrained clovibactin analogue retains moderate antibiotic activity, while a backbone N-methylated analogue is almost completely inactive. X-ray crystallography of these two analogues reveals that the macrolactone ring adopts a crown-like conformation that binds anions.
Conflict of interest statement
The authors declare the following competing financial interest(s): Initial support for work on clovibactin was provided by a subaward from NovoBiotic Pharmaceuticals LLC.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

